Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by relapsing eczema and pruritus. Until the development of Dupilumab, a new monoclonal antibody targeting IL-4 and IL-13 receptors, the current treatment of severe cases was based on immunosuppressant agents. Our main goal was to build a case series of five patients with severe atopic dermatitis, who were using immunosuppressive drugs with significant adverse effects and only partially controlled AD, and compare their symptoms, SCORAD index, treatment regimens, total and specific IgE, and blood cell count before and after the introduction of Dupilumab. SCORAD index and topical corticosteroids used on a daily basis had a significant decrease after 16 weeks of Dupilumab. Adverse effects were mild: conjunctivitis, local reaction and regional dermatosis. All patients with severe atopic dermatitis achieved better control of AD with Dupilumab than with immunosuppressive drugs. Adverse effects, secondary infections, total and specific IgE levels were greatly reduced.
Atopic dermatitis (AD) is a chronic inflammatory skin disease, characterized by relapsing intense pruritus, erythema, vesicular or maculopapular lesions, scaling, dry skin, crusts, and/or lichenification. The disease may have different grades of severity that can be estimated by specific tools, such as SCOring Atopic Dermatitis (SCORAD), Eczema Area and Severity Index (EASI), IGA. Superinfection by viruses, such as herpes simplex, including Kaposi’s varicelliform eruption, molluscum contagious and warts; or bacteria (Staphylococcus aureus) is frequent observed. AD prevalence, which is approximately 15% in children and 5% in adults, is increasing.1,2 Due to its chronic nature and frequent relapses, living with AD may involve a high burden, particularly for those requiring long-term, systemic treatment, since the immunosuppressants used can lead to serious adverse reactions. Pruritus and skin lesions may cause sleep disorder, anxiety, depression and low self-esteem, compromising the quality-of-life of patients and family.3 Severe cases are challenging for physicians, patients and their families.
Atopic dermatitis pathogenesis includes inflammation driven by a Th2 immune response, with sensitization to allergens, increased IgE levels, and blood eosinophilia; changes in skin barrier function, in some cases associated with mutations in the filaggrin gene; and increased colonization by Staphylococcus aureus. The systemic treatment most frequently used until recently for severe cases was one of the systemic immunosuppressants: cyclosporine, methotrexate, mycophenolate mofetil, and azathioprine. In 2017, the FDA fast-tracked the approval of Dupilumab for the treatment of moderate to severe patients with atopic dermatitis.3
Dupilumab is a fully human monoclonal antibody directly targeting the shared alpha chain of IL-4 and IL-13 receptors. These two cytokines are involved in the Th2 immune response, inducing allergen sensitization, promoting atopic inflammation, and decreasing the skin barrier function and structure.4 The monoclonal antibody inhibits the action of these cytokines and has been associated with gene expression changes in AD lesions, improving their molecular signature.5 This new therapeutic approach is based on AD pathogenesis and Dupilumab is changing the way we treat patients with moderate to severe AD, but more data with a greater number of patients and longer follow up in real life setting is in need. In this context, our main goal was to build a case series of five patients with severe atopic dermatitis who were using immunosuppressive drugs for long periods, with significant adverse effects and only partially controlled AD (Table 1). Aiming to reduce the adverse effects and improve the treatment efficacy, we switched patients’ therapy to Dupilumab after a transition time. This study has been carried out in accordance with The Declaration of Helsinki and all patients signed the informed consent. Complete clinical history with emphasis on topical and systemic treatment used, adverse effects, co-morbidities, worsening factors, number of secondary infections, physical examination and SCORAD index were performed, as well as total and specific IgE to major allergens and blood cell count before and after Dupilumab. All continuous variables were expressed as means±SD. Comparisons between two groups were made with the paired sample two-tailed Student’s t-test, and among three groups with RM one-way ANOVA.
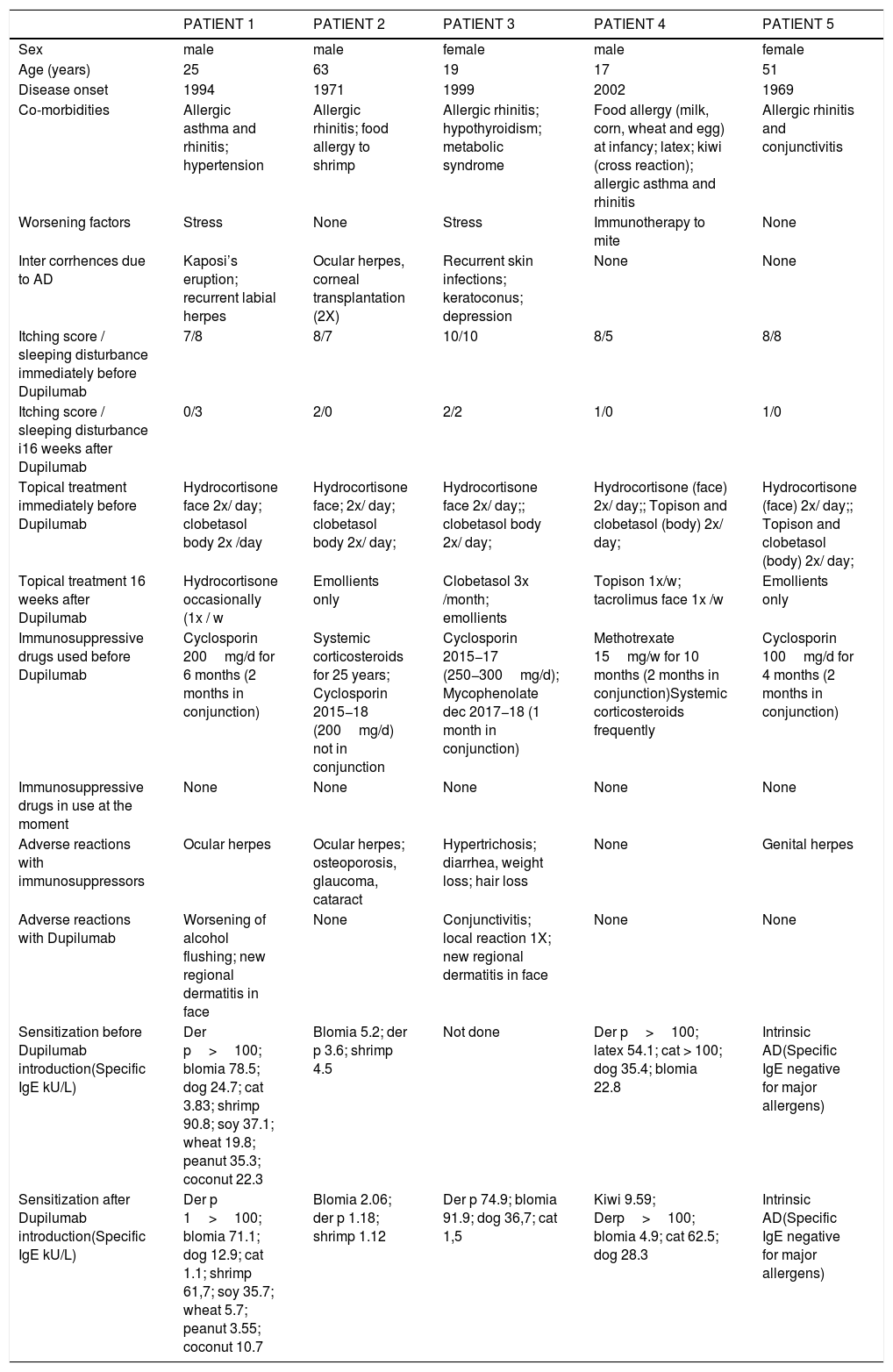
Patients’ clinical and laboratory characteristics before and after Dupilumab.
| PATIENT 1 | PATIENT 2 | PATIENT 3 | PATIENT 4 | PATIENT 5 | |
|---|---|---|---|---|---|
| Sex | male | male | female | male | female |
| Age (years) | 25 | 63 | 19 | 17 | 51 |
| Disease onset | 1994 | 1971 | 1999 | 2002 | 1969 |
| Co-morbidities | Allergic asthma and rhinitis; hypertension | Allergic rhinitis; food allergy to shrimp | Allergic rhinitis; hypothyroidism; metabolic syndrome | Food allergy (milk, corn, wheat and egg) at infancy; latex; kiwi (cross reaction); allergic asthma and rhinitis | Allergic rhinitis and conjunctivitis |
| Worsening factors | Stress | None | Stress | Immunotherapy to mite | None |
| Inter corrhences due to AD | Kaposi’s eruption; recurrent labial herpes | Ocular herpes, corneal transplantation (2X) | Recurrent skin infections; keratoconus; depression | None | None |
| Itching score / sleeping disturbance immediately before Dupilumab | 7/8 | 8/7 | 10/10 | 8/5 | 8/8 |
| Itching score / sleeping disturbance i16 weeks after Dupilumab | 0/3 | 2/0 | 2/2 | 1/0 | 1/0 |
| Topical treatment immediately before Dupilumab | Hydrocortisone face 2x/ day; clobetasol body 2x /day | Hydrocortisone face; 2x/ day; clobetasol body 2x/ day; | Hydrocortisone face 2x/ day;; clobetasol body 2x/ day; | Hydrocortisone (face) 2x/ day;; Topison and clobetasol (body) 2x/ day; | Hydrocortisone (face) 2x/ day;; Topison and clobetasol (body) 2x/ day; |
| Topical treatment 16 weeks after Dupilumab | Hydrocortisone occasionally (1x / w | Emollients only | Clobetasol 3x /month; emollients | Topison 1x/w; tacrolimus face 1x /w | Emollients only |
| Immunosuppressive drugs used before Dupilumab | Cyclosporin 200mg/d for 6 months (2 months in conjunction) | Systemic corticosteroids for 25 years; Cyclosporin 2015−18 (200mg/d) not in conjunction | Cyclosporin 2015−17 (250−300mg/d); Mycophenolate dec 2017−18 (1 month in conjunction) | Methotrexate 15mg/w for 10 months (2 months in conjunction)Systemic corticosteroids frequently | Cyclosporin 100mg/d for 4 months (2 months in conjunction) |
| Immunosuppressive drugs in use at the moment | None | None | None | None | None |
| Adverse reactions with immunosuppressors | Ocular herpes | Ocular herpes; osteoporosis, glaucoma, cataract | Hypertrichosis; diarrhea, weight loss; hair loss | None | Genital herpes |
| Adverse reactions with Dupilumab | Worsening of alcohol flushing; new regional dermatitis in face | None | Conjunctivitis; local reaction 1X; new regional dermatitis in face | None | None |
| Sensitization before Dupilumab introduction(Specific IgE kU/L) | Der p>100; blomia 78.5; dog 24.7; cat 3.83; shrimp 90.8; soy 37.1; wheat 19.8; peanut 35.3; coconut 22.3 | Blomia 5.2; der p 3.6; shrimp 4.5 | Not done | Der p>100; latex 54.1; cat > 100; dog 35.4; blomia 22.8 | Intrinsic AD(Specific IgE negative for major allergens) |
| Sensitization after Dupilumab introduction(Specific IgE kU/L) | Der p 1>100; blomia 71.1; dog 12.9; cat 1.1; shrimp 61,7; soy 35.7; wheat 5.7; peanut 3.55; coconut 10.7 | Blomia 2.06; der p 1.18; shrimp 1.12 | Der p 74.9; blomia 91.9; dog 36,7; cat 1,5 | Kiwi 9.59; Derp>100; blomia 4.9; cat 62.5; dog 28.3 | Intrinsic AD(Specific IgE negative for major allergens) |
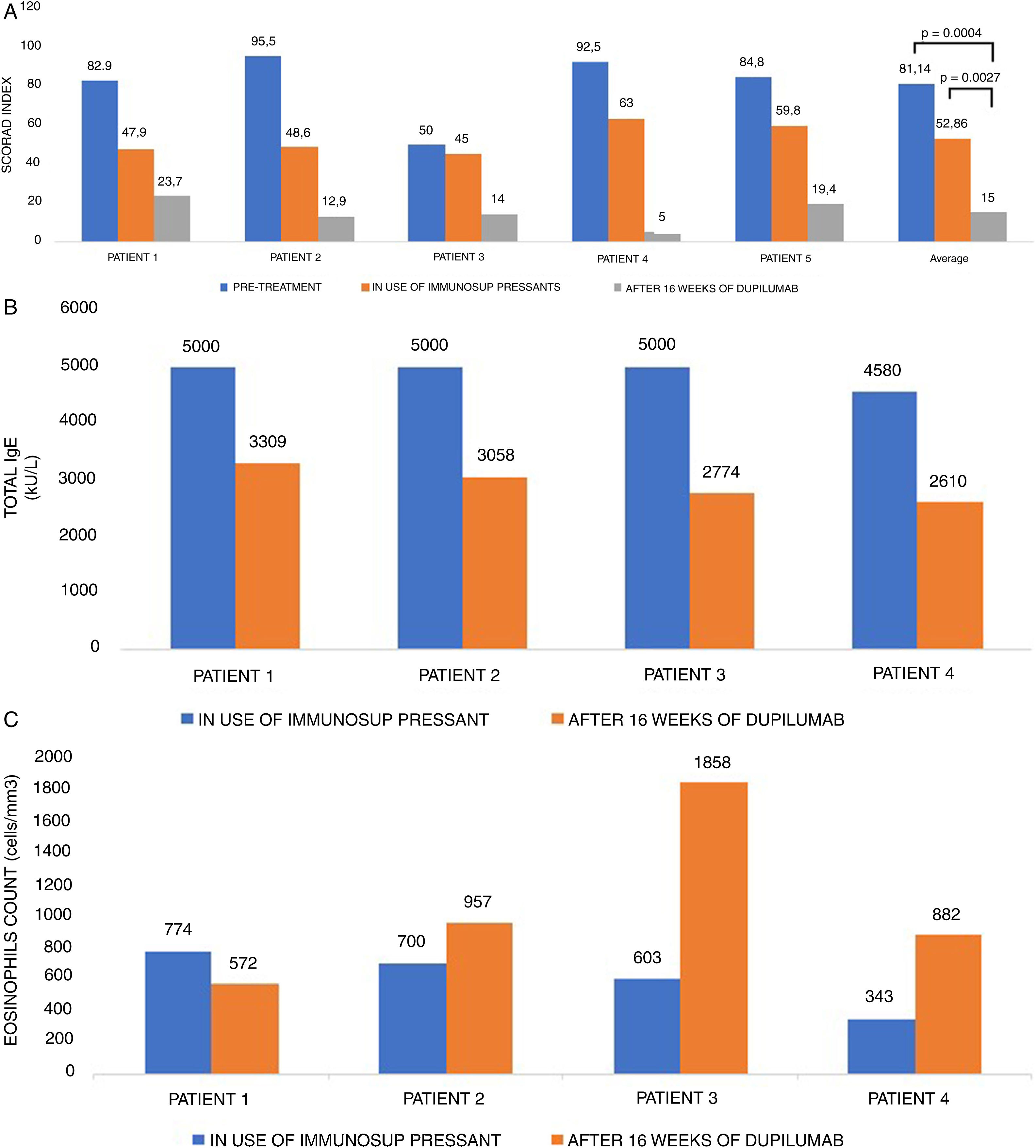
Table 1 contains all the information concerning the five cases. Patients treated were from different ages, 18–63 years old, with three males and two females. Their diseases were all of long duration, with a mean time of 31.8 years (17–50). Among the patients’ atopic comorbidities, 5/5 had allergic rhinitis, 2/5 had asthma, 2/5 had food allergy and 1/5 had allergic conjunctivitis. The worsening factors described by them were: stress in 2/5 and immunotherapy for mite in 1/5 patients. All patients were able to discontinue the immunosuppressant treatment within two months of the introduction of Dupilumab, after a transitional tapering phase. Regarding AD control, patients who already had shown some improvement with immunosuppressants in SCORAD (p=0.0147 comparing “pre-treatment” versus “in use of immunosuppressants”), had a much greater index reduction after 16 weeks of Dupilumab (Fig. 1A; p=0.0004 among the three groups: “pre-treatment”, “in use of immunosuppressants” and “in use of Dupilumab”; p=0.0027 between groups “in use of immunosuppressants” versus “in use of Dupilumab”). At that point, we could already observe the differences in the skin thickness (reduction of lichenification), along with all other signs of improvement in redness, excoriation, oozing, crusting and, specially, pruritus. The mean itching score of the patients before Dupilumab was 8.2 and after 16 weeks of treatment with Dupilumab was 1.2 (reduction of 85%). With regard to sleeping disturbances caused by atopic dermatitis in our five patients, the mean score before Dupilumab was 7.6 and after 16 weeks of treatment it was 1.0 (reduction of 87%). The amount and frequency of topical corticosteroids (TC) used on a daily basis also had a significant decrease (p<0.0001). During the immunosuppressant agent use, all patients had to apply two types of TC according to the body or face region twice a day, continuously, in the attempt to control their disease. After close to 16 weeks of Dupilumab use, the need for TC was sporadic since the disease was controlled. Our patients were feeling so much better and were so exhausted of all the years of TC that they would only use them if the disease had a relapse. Emollients, nevertheless, were used as maintenance therapy daily.
SCORAD (1A), total IgE level (kU/L) (1B), and eosinophils count (cells/mm3) (1C) in five patients with severe atopic dermatitis, pre-treatment, during the use of immunosuppressive drugs and after 16 weeks of Dupilumab treatment.
Obs: Patients with IgE above 5000 kU/L are marked as 5000, because the laboratory did not quantify higher levels.
Secondary infections were greatly reduced. Patient number 1 has had one episode of Kaposi’s varicelliform eruption and complained of recurrent labial and ocular herpes infection. Infection recurrence decreased from 12 to three episodes/year. Patient number 2 who had ocular herpes has showed no recurrences up until now. This is in accordance with a metanalysis of 2706 patients which showed that secondary infections decreased in the group of Dupilumab when compared to placebo. Patient number 3, who had secondary S. aureus skin infections about four times a year, had no more episodes since she started the new medication. She also had depression during her treatment with Mycophenolate Mofetil, probably not because of the drug itself, but because she had the worst period of AD control at that time associated with multiple adverse effects, such as diarrhea, weight loss and effluvium telogen. Her psychiatric treatment could be suspended after her improvement with Dupilumab.
Adverse reactions were mild (Table 1). One patient had conjunctivitis around the 10th week of Dupilumab treatment for a couple of months, which was successfully treated with corticosteroid drops. During the pivotal studies, conjunctivitis was the most frequent adverse reaction (8.6–22.1% versus 2.1–11.1% in the placebo groups).4 Herein, physicians must be aware of the signs, symptoms and treatment of conjunctivitis. In a real-life study with 241 patients, conjunctivitis was observed in 38.5% of the participants using Dupilumab.5 The development of conjunctivitis is significantly associated to a personal history of allergic conjunctivitis, and in the vast majority of cases is easily controlled with topical treatment.6 Three out of our five patients had new regional disease on the face, characterized by asymptomatic erythematous patches on the face and neck, which has been reported in the literature, but has a yet unknown pathologic mechanism.7 In one of them, the reaction started one year after the beginning of treatment and regressed spontaneously. In another patient, the new dermatosis of the face started three months after the introduction of Dupilumab and regressed after six months. The third patient complained of the new regional dermatosis after six months of the monoclonal antibody use, which improved with the use of topical hydrocortisone.
Initially, most of the patients had total IgE > 5000 kU/L (Fig. 1B). Total and specific IgE levels dropped in all patients, to around at least half of their initial values. Three patients showed transient eosinophilia for two months (Fig. 1C), which had not been reported in pivotal trials, but has already been published in real-world studies with Dupilumab in atopic dermatitis.5 Patient number 5 has intrinsic AD and also had disease clinical improvement with no adverse effects.
In conclusion, all five patients with severe atopic dermatitis achieved a much better control of AD with Dupilumab than with immunosuppressive drugs. The frequency and the severity of adverse reactions, secondary infections, and total and specific IgE levels were greatly reduced.