Sublingual immunotherapy (SLIT) for allergic rhinitis is a well-established and effective therapy, currently considered to be safer and almost equally effective alternative to classical subcutaneous immunotherapy (SCIT).
Regarding the safety of SLIT, recent meta-analyses and systematic reviews suggest a remarkably safe profile without any severe systemic reactions, anaphylaxis or use of adrenaline in 49 studies, reviewed by Radulovic et al.1 So far, only 11 cases of anaphylaxis to SLIT have been published.2–7
We present a case of a 35-year-old female suffering from persistent, perennial rhinoconjunctivitis and well-controlled asthma with seasonal aggravation. Symptoms appeared 15 years ago, and were becoming more severe each year, while symptomatic relief medications had been partly effective during the pollen season.
Sensitivity to Olea europaea pollen, Dermatophagoides pteronyssinus and Dermatophagoides farinae were identified by SPTs and serum s-IgE measurement. SLIT was carried out during the pollen season (in September) with standardised extracts of Sublivac (HAL, Netherlands) (10,000 allergy units/ml, 500AU/drop) Olea europaea and Sublivac D. pteronyssinus 50%, D. farinae 50%.
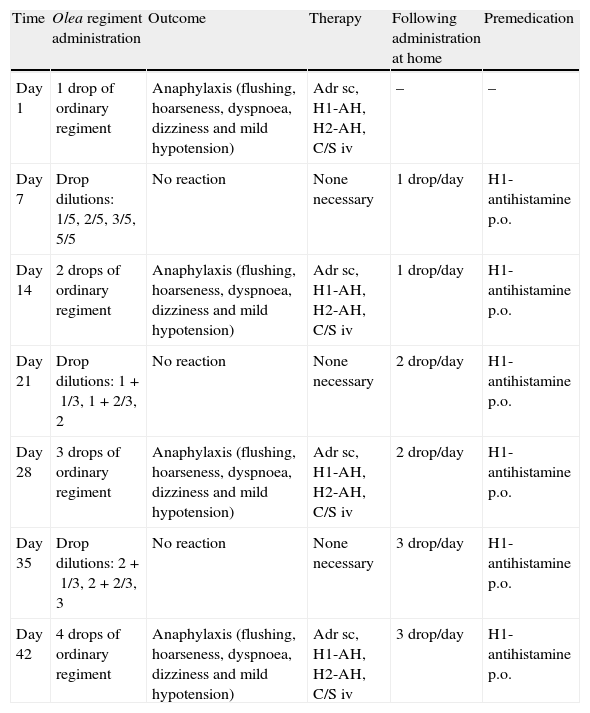
Induction of immunotherapy was initiated on two consecutive weeks. During the first week SLIT with HDM extract was advanced without any side-effect and the following week we proceeded to SLIT with Olea. The patient was administered the first drop of Olea extract and was attended in our department according to current guidelines. Within 10min, she developed an anaphylactic reaction (flushing, hoarseness, dyspnoea, dizziness and mild hypotension) and was treated with epinephrine, antihistamines and corticosteroids. In every attempt to increase the dose according to ordinary regiment she suffered a severe anaphylactic reaction (in all five reactions). Therefore, we adopted a modified build-up protocol (Table 1) and educated our patient to use autoinjectable adrenaline (no reaction occurred at home, where the dose remained unchanged).
Anaphylactic reactions to SLIT – modification of ordinary regiment.
| Time | Olea regiment administration | Outcome | Therapy | Following administration at home | Premedication |
| Day 1 | 1 drop of ordinary regiment | Anaphylaxis (flushing, hoarseness, dyspnoea, dizziness and mild hypotension) | Adr sc, H1-AH, H2-AH, C/S iv | – | – |
| Day 7 | Drop dilutions: 1/5, 2/5, 3/5, 5/5 | No reaction | None necessary | 1 drop/day | H1-antihistamine p.o. |
| Day 14 | 2 drops of ordinary regiment | Anaphylaxis (flushing, hoarseness, dyspnoea, dizziness and mild hypotension) | Adr sc, H1-AH, H2-AH, C/S iv | 1 drop/day | H1-antihistamine p.o. |
| Day 21 | Drop dilutions: 1+1/3, 1+2/3, 2 | No reaction | None necessary | 2 drop/day | H1-antihistamine p.o. |
| Day 28 | 3 drops of ordinary regiment | Anaphylaxis (flushing, hoarseness, dyspnoea, dizziness and mild hypotension) | Adr sc, H1-AH, H2-AH, C/S iv | 2 drop/day | H1-antihistamine p.o. |
| Day 35 | Drop dilutions: 2+1/3, 2+2/3, 3 | No reaction | None necessary | 3 drop/day | H1-antihistamine p.o. |
| Day 42 | 4 drops of ordinary regiment | Anaphylaxis (flushing, hoarseness, dyspnoea, dizziness and mild hypotension) | Adr sc, H1-AH, H2-AH, C/S iv | 3 drop/day | H1-antihistamine p.o. |
In-hospital attempts to increase dose during build-up phase, outcomes and self-administered doses at home the subsequent days.
Dilutions of ordinary regiment were prepared using saline and administered in 30min intervals.
AH, antihistamines.
The step-up process with SLIT to olive was quite difficult to advance because she could not tolerate more than three drops of extract per day. On the contrary, SLIT to House Dust Mites advanced with no reaction, and the maintenance dose was reached in five days.
Although our patient did not have to withdraw from SLIT, she eventually remained on a lower maintenance dose (3drops/day), because she had got tired and refused to consent to any subsequent dose increase. That maintenance dose proved to be effective, as she reported a 50% decline on symptom-medication score during the next pollen season.
Despite the fact that the safety of SLIT is well-established, adverse reactions may occur, mainly during the build-up phase. Furthermore all previously published reports of anaphylaxis during SLIT, were characterised either by a deviation from international guidelines,1,3 or by previous frequent reactions during SCIT.4,6
In our case, anaphylactic episodes occurred in-hospital following the first dose and also after any subsequent dose increase, in a young female asthmatic patient lacking any history of anaphylaxis. During the build-up phase, asthma was well controlled and no predicting factor for a subsequent anaphylaxis was present. Despite these reactions, we managed to increase the tolerated dose, by administering premedication (antihistamine p.o.) during the build-up phase, increasing gradually the dose only in-hospital and maintaining the same dose for seven consecutive days at home (Table 1).
The remarkable safety profile of SLIT allowed the recent modification of SLIT schedules, omitting the up-dosing phase and starting with the maintenance dose. However, it seems that there is a subgroup of sensitive patients who need a more conservative build-up schedule in order to avoid a severe anaphylactic reaction.
Although SLIT seems to have an excellent safety profile this does not rule out the possibility of an anaphylactic reaction even if current guidelines have been followed precisely. It is imperative to administer the first dose under medical supervision and be vigilant to immediately recognise any possible systemic reaction. Our case indicates that SLIT schedules with a large dose of allergens, omitting up-dosing phase, may not be safe enough for a minority of high sensitive patients to whom a more gradual dose increase could be necessary. So far, the majority of studies focus mainly on effectiveness of SLIT and, to a lesser extent, on side-effects (usually considered as secondary outcomes). Therefore, more studies primarily focused on adverse reactions to SLIT are needed.1
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.