A clear picture of interaction of Th1/Th2 cytokines in pathogenesis of chronic spontaneous urticaria (CSU), remains elusive. Impaired IFN-γ production and decreased levels of IL-2 have been reported. The aim of this study was to evaluate the association of Th1 cytokines; IL-2, IL-12 and IFN-γ polymorphisms with CSU.
Methods90 patients with CSU and 140 age-sex matched subjects were included in this study. DNA samples were evaluated through PCR-SSP assay in order to detect single nucleotide polymorphisms of IL-12 (A/C −1188) or (rs3212227), IFN-γ (A/T UTR5644) or (rs2069717) and IL-2 (G/T −330 and G/T +166) or (rs2069762 and rs2069763).
ResultsG allele at −330 at promoter region of IL-2 gene was overrepresented in CSU. Heterozygotes (GT) at this locus and heterozygotes at +166 of IL-2 gene (GT) were more prevalent in CSU group. Additionally, the haplotype GT for loci −330 and +166 of IL-2 gene was powerfully associated with CSU (OR (95%CI)=57.29 (8.43–112.7)).
ConclusionsSNP at position −330 and +166 of IL-2 gene are differently expressed in CSU. The haplotype GT of IL-2 at −330 and +166 might confer vulnerability to a number of immunological disorders in Iranian region.
Recurrence or persistence of pruritic erythematous wheals or angio-edema for at least six weeks is designated as chronic urticaria.1 Chronic spontaneous urticaria (CSU) is a term that has now replaced the two terms of chronic idiopathic urticaria (CIU) and chronic autoimmune urticaria (CAU), the later characterized by positive serum anti-Fc¿R1β and/or anti-IgE auto antibodies.2 Patients with CSU have a lower quality of life; experience more of symptoms of depression, anxiety and sleep difficulties and also have reduced working capacity.3 A strong correlation exists between the activity of CSU, determined by urticaria activity score 7, and quality of life scores, determined by chronic urticaria quality of life questionnaire (CU-Q2oL).4 Spontaneous urticaria was first devised to describe occurrence of spontaneous wheals or angio-edema for no known external cause apparent at clinical examination.5 While all types of urticaria can be chronic in nature, physical urticarial to name some, the term chronic spontaneous urticaria has now replaced the former term chronic idiopathic urticaria, to rightfully represent the chronic nature and unknown eliciting factor of this subgroup of urticaria.5 A wide array on underlying predisposing factors have been sub-classified or identified as causative factors in chronic urticaria, among which the role of functional auto-antibodies and chronic infections have been emphasized.5 Autoimmune urticaria, for instance was formerly classified as a unique entity, by the presence of high affinity IgE receptor antibodies. Meanwhile, in as much as 50% of sera of patients with CSU, high affinity auto-anti Fc¿R1, anti-IgE antibody or mast-cell activating non-immunoglobulin mediators can be detected,6 and a positive autologous serum skin test (ASST) proves an immunological substrate in 25–45% of CSU (former CIU) patients.7
A powerful association has been found between FCER gene (FC epsilon receptor gene 1) α and β chains, polymorphisms, with CSU.8,9 The high-affinity IgE receptor, FC¿ receptor 1, on basophils and mast cells is the primary site of action for activating auto-antibodies to cross-link the receptors and initiate a response. This mechanism is however confined to patients with high total IgE (RIST assay) or patients with positive ASST test. There is a mixed and heterogeneous cytokine network of both Th1 and Th2 response in most patients with CSU, and the spontaneous nature of CSU justifies this heterogeneity. In fact an individual immune profile might underlie each case of CSU.10 sIL-2R and TNF-α concentrations are higher in serum of atopic patients with CSU, while the Th2 profile cytokines are the prevailing cytokines in patients with chronic spontaneous angio-edema.10
We have recently shown that single nucleotide polymorphisms (SNPs) of pro-inflammatory cytokines such as IL-6 and TNF-α1 as well as IL-10 and TGF-β are associated with CSU.11 Although the association of Th1 cytokines with several diseases has been investigated,12–17 it has not been studied in CSU. Herein, the possible association of IL-2, IL-12 and IFN-γ SNPs with CSU was studied in order to elucidate the underlying immune dysregulation in patients suffering from CSU.
Materials and methodsStudy designThis case–control study comprised a number of 90 patients (75 females) with CSU, who were selected by simple randomization from referrals to the Children's Medical Center, the Pediatrics Center of Excellence in Tehran, Iran. The diagnosis of Chronic Urticaria was primarily made with a history of at least two recurrences of wheals and/or angio-edema in a weak that lasted for six weeks or more, according to standard international guidelines.18 To ascertain the spontaneous nature of urticaria, detailed history including the time of onset, duration, characteristics and distribution of lesions and also history of associated illnesses, food or drug allergy was taken. Subjects with any sign of physical urticaria, cold urticaria, urticarial vasculitis and food or drug induced urticaria were excluded from the patients group. Finally, specific laboratory tests were performed; including C3, C4, CH50, and C1-inhibtor (C1-INH) in order to exclude patients with complement deficiencies. Additionally, a number of 140 age-sex matched subjects with no evidence of allergic or autoimmune disorders were randomly selected as control group from the control bank of our research center.19 This study was approved by the local ethics committee and institutional review board of Tehran University of Medical Sciences. The participants were provided with detailed information about the aim and protocol of the study and signed informed consent forms.
Sampling and genotypingThe amount of 5ml of whole blood was taken from all participants and preserved with ethylene-diamine-tetra-acetic acid (EDTA) until investigation. Genomic DNA was extracted from peripheral blood mono-nucleated cells using phenol–chloroform method.20 The extracted DNA was amplified using a PCR Techne Flexigene apparatus (Roche, Cambridge, UK) as explained before.12 Polymerase chain reaction with the Sequence Specific Primers (PCR-SSP) assay was employed (PCR-SSP kit, Heidelberg University, Heidelberg, Germany) for SNP detection. PCR products were observed by 2% agarose gel under a UV transilluminator. SNP's at positions: IL-12 (A/C −1188) or (rs3212227), IFN-γ (A/T UTR5644) or (rs2069717) and IL-2 (G/T −330, G/T +166) or (rs2069762, rs2069763) were targeted for evaluation.
Statistical analysisThe analyses were all performed using the Epi Info statistical software (version 6.2, World Health Organization, Geneva, Switzerland). The proper sample size was calculated according to an earlier publication21 with α=0.05, β=0.2 (power=80%) and 3:2 as control–case ratio. Allele, genotype and haplotype differences were estimated by direct gene counting. The counts of patients and controls group were compared using chi-square test and odds-ratios with 95% confidence interval (95%CI) were estimated. All tests were two-sided and the probability of less that 0.05 was considered as statistically significant.
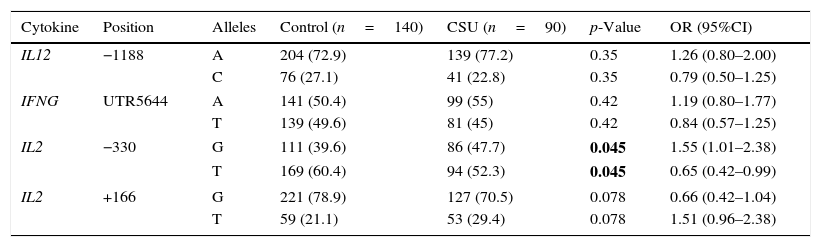
ResultsAllelic differencesSNP analysis of patients with CSU revealed no significant difference in allele frequencies of IL-12 (A/C, −1188) and IFN-γ (A/T, UTR5644) between cases and controls. The G allele at IL-2, −330 was significantly more prevalent in patients with CSU compared to control group (p-value=0.045) (Table 1).
Allele differences of IL-2, IL-12 and IFN-γ in patients with CSU and controls.
| Cytokine | Position | Alleles | Control (n=140) | CSU (n=90) | p-Value | OR (95%CI) |
|---|---|---|---|---|---|---|
| IL12 | −1188 | A | 204 (72.9) | 139 (77.2) | 0.35 | 1.26 (0.80–2.00) |
| C | 76 (27.1) | 41 (22.8) | 0.35 | 0.79 (0.50–1.25) | ||
| IFNG | UTR5644 | A | 141 (50.4) | 99 (55) | 0.42 | 1.19 (0.80–1.77) |
| T | 139 (49.6) | 81 (45) | 0.42 | 0.84 (0.57–1.25) | ||
| IL2 | −330 | G | 111 (39.6) | 86 (47.7) | 0.045 | 1.55 (1.01–2.38) |
| T | 169 (60.4) | 94 (52.3) | 0.045 | 0.65 (0.42–0.99) | ||
| IL2 | +166 | G | 221 (78.9) | 127 (70.5) | 0.078 | 0.66 (0.42–1.04) |
| T | 59 (21.1) | 53 (29.4) | 0.078 | 1.51 (0.96–2.38) | ||
n, denotes number of participants in each group. Bold values signify p<0.05.
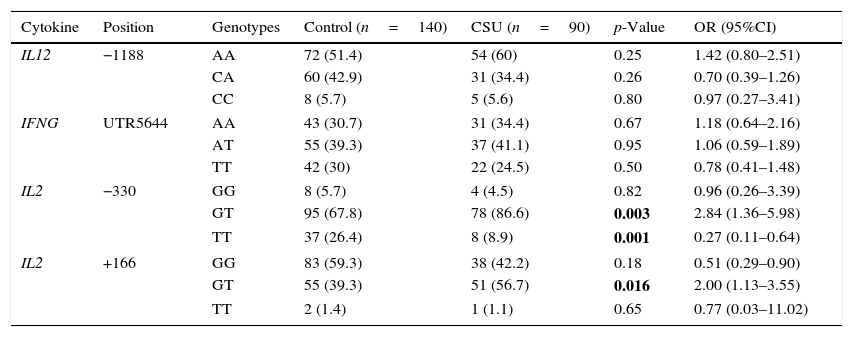
Heterozygotes at position −330 of IL-2 gene were more likely to have CSU (OR (95%CI)=2.84 (1.36–5.98)). The same result attributed to the heterozygotes at position +166 (OR (95%CI)=2.00 (1.13–3.55)). Consistent with lower frequency of T allele at position −330, CSU was less common in carriers of the TT genotype (OR (95%CI)=0.27 (0.11–0.64)) (Table 2).
Genotype differences of IL-2, IL-12 and IFN-γ in patients with CSU and controls.
| Cytokine | Position | Genotypes | Control (n=140) | CSU (n=90) | p-Value | OR (95%CI) |
|---|---|---|---|---|---|---|
| IL12 | −1188 | AA | 72 (51.4) | 54 (60) | 0.25 | 1.42 (0.80–2.51) |
| CA | 60 (42.9) | 31 (34.4) | 0.26 | 0.70 (0.39–1.26) | ||
| CC | 8 (5.7) | 5 (5.6) | 0.80 | 0.97 (0.27–3.41) | ||
| IFNG | UTR5644 | AA | 43 (30.7) | 31 (34.4) | 0.67 | 1.18 (0.64–2.16) |
| AT | 55 (39.3) | 37 (41.1) | 0.95 | 1.06 (0.59–1.89) | ||
| TT | 42 (30) | 22 (24.5) | 0.50 | 0.78 (0.41–1.48) | ||
| IL2 | −330 | GG | 8 (5.7) | 4 (4.5) | 0.82 | 0.96 (0.26–3.39) |
| GT | 95 (67.8) | 78 (86.6) | 0.003 | 2.84 (1.36–5.98) | ||
| TT | 37 (26.4) | 8 (8.9) | 0.001 | 0.27 (0.11–0.64) | ||
| IL2 | +166 | GG | 83 (59.3) | 38 (42.2) | 0.18 | 0.51 (0.29–0.90) |
| GT | 55 (39.3) | 51 (56.7) | 0.016 | 2.00 (1.13–3.55) | ||
| TT | 2 (1.4) | 1 (1.1) | 0.65 | 0.77 (0.03–11.02) | ||
n, denotes number of participants in each group. Bold values signify p<0.05.
The haplotype GT of IL-2 at positions −330 and +166, was significantly more common in patients with CSU (OR (95%CI)=57.29 (8.43–112.7)) (Table 3).
Haplotype differences of IL-2, IL-12 and IFN-γ in patients with CSU and controls.
| Cytokine | Haplotype | Controls (n=140) | CSU (n=90) | p-Value | OR (95%CI) |
|---|---|---|---|---|---|
| IL2 (−330, +166) | GG | 109 (38.9) | 62 (34.4) | 0.07 | 0.71 (0.49–1.03) |
| TG | 112 (40) | 65 (36.2) | 0.053 | 0.69 (0.48–1.01) | |
| TT | 57 (20.4) | 29 (16.1) | 0.91 | 0.95 (0.61–1.49) | |
| GT | 2 (0.7) | 24 (13.3) | <0.001 | 57.29 (8.43–112.7) |
n, denotes number of participants in each group. Bold values signify p<0.05.
Despite the overall increase in skin resident and circulating CD4+ T-cells,22 CD8+ cytotoxic T-cells are the main source of IL-4 production in patients with CSU.23 Also, while serum levels of both IL-4 and IFN-γ are elevated in CSU,24 stimulated IFN-γ production is commonly suppressed in both Th1 and Tc1 cells specially in the atopic subgroup of CSU patients.22 This might be a result of a late cytokine milieu, inducing tolerance by phenotypic differentiation of B-cells and cytotoxic T-cells into regulatory cells and by suppressing the initiation of both IL-4 and IFN-γ response in plasmacytoid dendritic cells.25
Increased IL-10 production by helper and cytotoxic T-cells is perhaps the underlying mechanism for inhibition of formation of a full pictured Th1 or Th2 response.22 IL-10 is also responsible for reduced IL-2 expression, and the idea has risen that if we are able to enhance the production of IL-2 and IFN-γ and promote the Th1 response, while suppressing IL-10 levels, we could treat CSU.26 Nonetheless, as Th1 is the dominant response in the atopic CSU patients, IL-10 suppression might not eliminate the underlying pathology of CSU.
The GG genotype at IL-2 gene, −330 is a high yield producer of IL-2 as demonstrated by better in vitro activation of T-cells and NK cells carrying GG genotype,27 while the T allele and the TT genotype are considered low yield IL-2 producers. Herein we found a lower prevalence of CSU in carriers of T allele and TT genotype at this position. Moreover, the T allele at this position, confers lower risk of systemic lupus erythematosus (SLE) in our region.28 Although, not all CSU case have autoimmune components, a striking association exists between CSU and autoimmune disorders like SLE29 and autoimmune thyroiditis with Grave's disease, being mostly investigated.30 Chronic urticarial rash is a common skin finding in SLE patients with a comorbidity up to 21.9–27.5%.29 In fact, the term “autoimmunity-related neutrophilic dermatosis” has been proposed to distinguish subjects with CSU in the context of autoimmune connective tissue disorders while ruling out urticarial vasculitis as it is commonly misdiagnosed as CSU in these patients. Analysis of autoantibodies and their subtypes in large groups of patients with SLE and CSU has revealed that mast cell activating IgG3 and IgG3 auto anti-dsDNA antibodies are found far more commonly than it was thought is CSU patients, and similar pathomechnisms underlie CSU and SLE.29 The T allele, which is low is associated with lower risk of (SLE).28
The GG genotype of IL-2 at −330 is similarly associated with excessive IL-2 production and high risk of developing Graves’ disease.31 According to Ruggeri et al., the overlapping array of circulating auto antibodies (up to 43% of CSU patients have thyroid autoantibodies), can explain the autoimmune nature and concordance of Grave's and CSU.30 While pruritus and recurrent wheals are a very common cutaneous complaint in Grave's thyrotoxic patients, symptoms frequently resolve, upon establishment of a euthyroid state.32 The G allele at this position, was similarly associated with higher CSU in our population.
Conclusively, carriers of the TT genotype are low yield producers of IL-2 in terms of transcription/translation level of the respective mRNA/protein, while the GG genotype confers higher ability to produce IL-2. Meanwhile, the IL-2 −330 site is located in close proximity to the nuclear factor of activated T-cells (NFAT). Therefore, polymorphisms of this position at IL-2 gene might also affect IL-2 transcription and translation, by regulating the activity of this transcription factor.33
Interestingly, 17% of patients carry the GT haplotype of IL-2 gene at −330 and +166, compared to only 0.3% of controls, this variant could be considered a robust predictor of CSU (OR (95%CI)=57.29 (8.43–112.7)). We had previously found an association between this haplotype and juvenile idiopathic arthritis and systemic lupus erythematosus in our region.28,34
Any conclusion from this study is limited by the low number of enrolled cases, which lead to low power of the study.
ConclusionSNP at position −330 and +166 of IL-2 gene are differently expressed in CSU. The haplotype GT of IL-2 at −330 and +166 might confer vulnerability to a number of immunological disorders in Iranian region.
Ethical disclosuresConfidentiality dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestNone declared.
This study was supported by a grant from Tehran University of Medical Sciences (25155).