T cells play an important role in bronchial asthma. Although airway CD4+ T cells have been extensively studied previously, there are hardly any studies relating CD8+ T cell activation and disease symptoms.
ObjectivesThe aim of this study was to analyse the association between T cell activation in induced sputum T cells and asthma severity and control; and to evaluate T cell subpopulations in the same subgroups.
MethodsFifty allergic asthmatic patients were recruited and lung function testing was performed. Airway cells were obtained by sputum induction via inhalation of hypertonic saline solution. CD3, CD4, CD8, CD28, CD25 and CD69 were studied by flow cytometry in whole induced sputum and peripheral blood cells.
ResultsTotal induced sputum T cells and CD8+ T cells had a higher relative percentage of the activation markers CD25 and CD69 in comparison with peripheral blood. In sputum, the relative percentage of CD25 was higher in CD4+ T cells when compared to CD8+ T cells and the reverse was true regarding CD69. However, neither disease severity nor control were associated with the relative percentage of CD25 or CD69 expression on T cells in sputum.
ConclusionsBoth CD4+ and CD8+ T cells are activated in the lungs and peripheral blood of asthmatic patients. However, with the possible exception of CD69+ CD8+ T lymphocytes in the sputum, there is no association between T cell activation phenotype in the target organ and disease severity or control.
Asthma is characterised by variable airflow obstruction, airway hyperresponsiveness, and chronic airway inflammation, involving activated eosinophils, mast cells, and T lymphocytes.1 It is known that airway inflammation is a feature of asthma. Typically, airway inflammation has been assessed by bronchial biopsies or bronchoalveolar lavage fluid (BAL). Examination of spontaneous or induced sputum has advantages over these invasive methods, as it is inexpensive, easy to perform, well-tolerated, non-invasive, safe, and can be performed repetitively.2 The cellular composition of sputum correlates well with that of BAL, but to a lesser extent with that of bronchial biopsies.3,4 In addition to cellular sputum composition, there is increasing interest in the analysis of cellular subtypes and activation, which can be performed using flow cytometry.5
Traditionally, type 2 CD4+ T cells (Th2), have been regarded as the most important T cell subset in the pathophysiology of asthma.6 CD8+ T cells are considered to be of lesser importance in bronchial asthma. However, increased numbers of activated CD8+ T cells in the airways may cause acute tissue damage via the release of lytic substances, such as perforin and granzymes. In this regard, in chronic obstructive pulmonary disease (COPD), also an inflammatory lung disease, CD8+ T cells contribute to the abnormal inflammatory process, and play an important role in the pathogenesis of disease. CD8+ T cells can be subdivided into functional and phenotypically different populations, on account of CD28 expression.7 CD28 is a co-stimulatory receptor, constitutively expressed on approximately 95% of CD4+ T cells and 50% of CD8+ T cells in humans, and is crucial for the activation and proliferation of naive T cells.8 CD8+CD28+ T cells are predominant in young healthy individuals and CD8+CD28− cells increase with ageing and during inflammation and autoimmune disease.9 It was suggested that CD8+ T cells can contribute to the pathology of asthma death due to an enhancement of pre-existing airway inflammation in response to viral infection.10,11 Furthermore, human airway CD8+ T cells spontaneously produce increased type 1 and type 2 cytokines in subjects with asthma, when compared to healthy controls.12
Both CD4 and CD8+T cells can express CD25, the interleukin-2 receptor α-chain, which is a marker of both T cell activation and T cell proliferation. Both cell types, apart from macrophages, eosinophils and neutrophils, may also express the activation marker CD69.
Airway inflammation in asthma can have variable intensity and biological features and, to a certain extent, may have a relationship to disease severity.13 In fact, asthma guidelines recommend stepwise increments in anti-inflammatory medication to control increased disease activity.14 However, few studies have analysed the relationship between disease severity and parameters of T cell activation.
The aims of this study were to compare T cell subpopulations and activation status between peripheral blood and induced sputum in asthma, and to correlate relative percentages in induced sputum with severity and control of the disease.
Materials and methodsVolunteersFifty adult allergic asthmatics were recruited from the Allergy Clinic of the Cova da Beira Hospital and lung function testing was performed. Asthma was defined according to the American Thoracic Society criteria.15 Allergy was confirmed by clinical history, skin prick testing, and determination of total and specific IgE. Asthma severity was assessed at the first appointment, according to the Global Initiative for Asthma (GINA) guidelines.16 According to the GINA guidelines asthma severity should be classified in the first appointment according to: symptoms; amount of beta2-agonists needed to treat symptoms; and lung function. Both the level of airflow limitation and its variability enable asthma to be subdivided by severity into four steps: Intermittent, Mild Persistent, Moderate Persistent, and Severe Persistent.
Asthma control was assessed by the Asthma Control Test (ACT),17 just before the induction of sputum. Controlled classes were merged, since the important comparison in the study was between uncontrolled (score of less than 20 points in ACT) and any kind of controlled patients (score equal to or above 20).
None of the patients had changes in the medication or respiratory infections in the month prior to the induction, and none was on oral corticosteroids.
Twelve healthy volunteers were also recruited among the personnel of the Cova da Beira Hospital.
The study was approved by the Hospital Ethics Committee and all subjects gave written informed consent. The procedures are in accordance with the World Health Association and the Helsinki Declaration.
Sputum inductionAll patients were given detailed information and clear instructions prior to the procedure. Before sputum induction, all patients inhaled 12μg formoterol via a metered dose inhaler.
Sputum was induced and processed according to the method described by Pizzichini et al.,3 with minor modifications.
Baseline peak expiratory flow (PEF) was measured prior to induction, and the measurement was repeated following formoterol, and after each 5 minute inhalation of nebulised hypertonic saline (5%). The procedure was stopped if PEF fell by >20% at any time, or if bothersome symptoms occurred. After each period of inhalation, volunteers were asked to rinse their mouth with water and blow their nose, and attempt to cough sputum into a sterile container. The cumulative duration of nebulisation was 15–20min.
Sputum processingUnselected sputum was weighed and 0.1% dithiothreitol (DTT, Sigma-Aldrich, Saint Louis, MO, USA) in phosphate buffered saline (PBS) was added at a ratio of 4ml to 1g sputum. The sputum was incubated with DTT at room temperature for 15min on a rolling mixer. The same volume of PBS (4ml to 1g sputum) was added to the mixture and incubated for another 10min. The suspension was then filtered through a 40μm cell strainer (Falcon). The filtrate was centrifuged at 500g for 10min at room temperature to pellet cells. After two washing steps, cells were resuspendend in PBS and viability was determined by tryptan blue (Sigma-Aldrich) exclusion staining in a Neubauer haemocytometer (Merck Eurolabs, Lutterworth, UK).
PBMC preparationPeripheral blood mononuclear cells (PBMC) were separated from 20ml heparinised peripheral blood using Lymphoprep (Nycomed, Oslo, Norway), washed twice in RPMI 1640 (Sigma Aldrich) without additives, and counted.
Flow cytometrySputum cells and PBMC were washed in PBS containing 0.1% sodium azide (Sigma-Aldrich) and 0.4% bovine serum albumin (Sigma-Aldrich) (staining medium) and centrifuged at 300g. For each test, approximately 500,000 lymphocytes were incubated with fluorochrome stained antibodies for 30min at 4°C in the dark. Cells were then washed, resuspended in PBS and acquired using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). Up to 20,000 CD3 positive events were collected per sample. All samples were stained with an APC-conjugated anti-human CD3 monoclonal antibody (mAb), FITC conjugated anti-human CD4 mAb, and PerCP conjugated anti-human CD8 mAb. To assess surface marker expression, samples were incubated with anti-CD25, -CD69, or -CD28 PE-conjugated mAbs (Becton Dickinson). Isotype-matched antibodies were used as controls. The number of positive cells for each surface marker was expressed as a percentage of CD4+ or CD8+ cells. In the specific case of CD25, all events which expressed a higher MFI for CD25 than the blank sample, (and not only those with the highest intensity of expression) were considered positive.
DTT tests in peripheral bloodAs sputum has to be homogenised before flow cytometry, a possible effect of DTT treatment on surface markers has to be considered. However, in contrast to peripheral blood leukocytes, induced sputum leukocytes can only be obtained with DTT treatment.
In order to evaluate the influence of DTT on surface for the markers under study, we performed the same procedure of sputum processing using peripheral blood. Half the sample was treated with DTT and the other half with PBS alone in the same proportion as that of sputum (1g peripheral blood: 4ml DTT or 4ml PBS). Afterwards PBMC were isolated and stained as described.
Data analysisFlow cytometry data were acquired and analysed using CellQuest Software (Becton Dickinson). Data are expressed as median and range. Wilcoxon signed rank test was used for comparison within the same group, and Mann-Whitney U test was used for comparison between two groups. A value of p<0.05 was considered significant.
Statistical analyses were performed using Minitab 14.
ResultsCharacteristics of patientsFifty allergic asthmatic patients were recruited for this study.
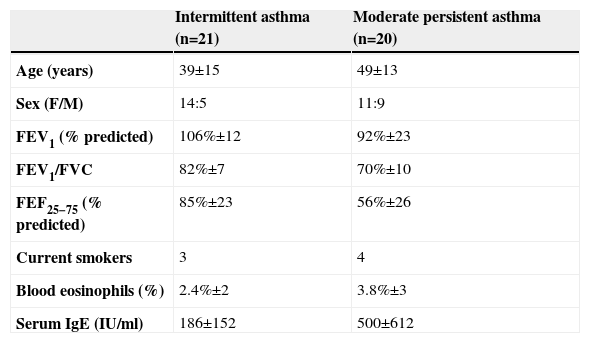
Sputum was successfully induced in 45 patients; however, flow cytometry could only be performed in 41 of the samples due to insufficient number of T cells. Patients with different asthma severities were paired regarding age and gender. Twenty one patients (age 39±15, 5 males) had intermittent asthma and were not under inhaled corticosteroids. They only resorted to rescue medication with short-acting β2-agonists. Twenty patients had moderate persistent asthma (age 49±13, 9 males); all were receiving beclomethasone dipropionate or equivalent (budesonide or fluticasone) daily (median dose 500μg per day), 11 of them were also receiving long-acting β2-agonists, and 6 were on anti-leukotrienes. In the intermittent asthma group FEV1 was 106%±12, FEV1/FVC was 82±7 and FEF 25-75 was 85%±23. In the moderate persistent asthma group FEV1 was 92%±23, FEV1/FVC was 70±10 and FEF 25–75 was 56%±26. Patients were paired for smoking (three in the intermittent group and four in the moderate persistent group were smokers) (Table 1).
Clinical characteristics of study subjects
| Intermittent asthma (n=21) | Moderate persistent asthma (n=20) | |
| Age (years) | 39±15 | 49±13 |
| Sex (F/M) | 14:5 | 11:9 |
| FEV1 (% predicted) | 106%±12 | 92%±23 |
| FEV1/FVC | 82%±7 | 70%±10 |
| FEF25−75 (% predicted) | 85%±23 | 56%±26 |
| Current smokers | 3 | 4 |
| Blood eosinophils (%) | 2.4%±2 | 3.8%±3 |
| Serum IgE (IU/ml) | 186±152 | 500±612 |
Results are expressed as mean (±SE).
FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; FEF=forced expiratory flow.
Regarding the healthy volunteers, no data are presented as we were only able to collect enough T cells on two occasions.
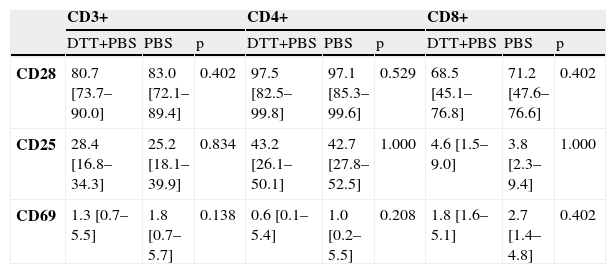
Sputum processingSince DTT may affect results with sputum T cells, we also performed DTT incubations with peripheral blood. No differences were seen in staining of any of the cell surface markers (CD28, CD25 or CD69) on CD3+, CD4+ or CD8+ cells between peripheral blood samples pre-treated with DTT plus buffer (PBS), and those treated with buffer alone (Table 2).
The effect of dithiothreitol (DTT) on the percentage of blood lymphocytes expressing CD28, CD25 and CD69 (n=6)
| CD3+ | CD4+ | CD8+ | |||||||
| DTT+PBS | PBS | p | DTT+PBS | PBS | p | DTT+PBS | PBS | p | |
| CD28 | 80.7 [73.7–90.0] | 83.0 [72.1–89.4] | 0.402 | 97.5 [82.5–99.8] | 97.1 [85.3–99.6] | 0.529 | 68.5 [45.1–76.8] | 71.2 [47.6–76.6] | 0.402 |
| CD25 | 28.4 [16.8–34.3] | 25.2 [18.1–39.9] | 0.834 | 43.2 [26.1–50.1] | 42.7 [27.8–52.5] | 1.000 | 4.6 [1.5–9.0] | 3.8 [2.3–9.4] | 1.000 |
| CD69 | 1.3 [0.7–5.5] | 1.8 [0.7–5.7] | 0.138 | 0.6 [0.1–5.4] | 1.0 [0.2–5.5] | 0.208 | 1.8 [1.6–5.1] | 2.7 [1.4–4.8] | 0.402 |
There were significant differences in the CD4/CD8 ratio between sputum and peripheral blood (3.6 vs. 2.0, respectively, p=0.005). This difference was due to a decrease in the levels of CD8+ T cells in induced sputum (32.0% vs. 19.5%, p=0.001), with no difference in CD4+ T cell levels (63.0% vs. 73.5%, p=0.056).
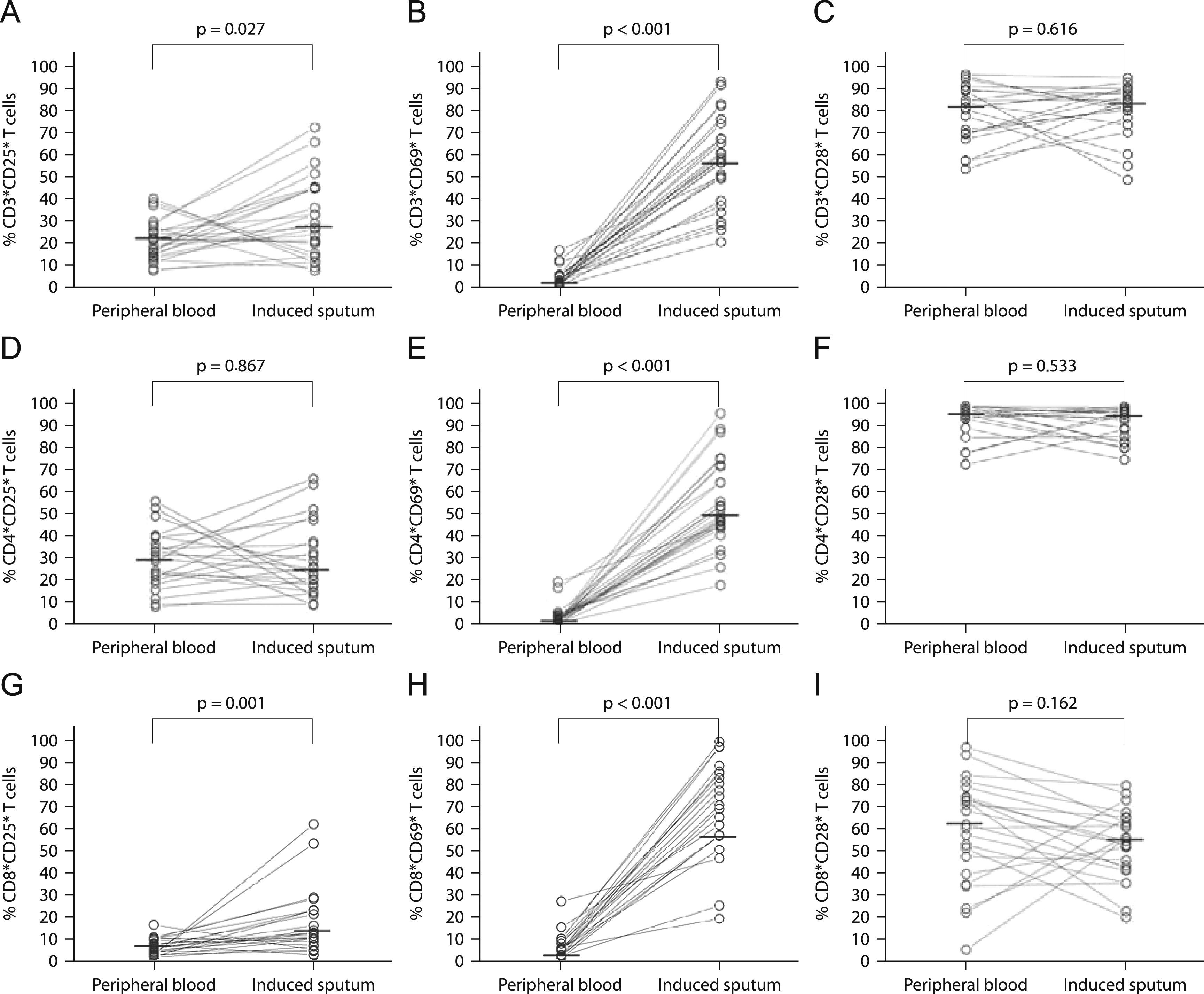
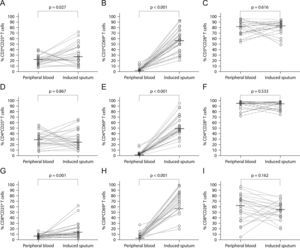
In the present study, there were no significant differences in relative percentages of CD8+CD28+ and CD8+CD28− T cells between peripheral blood and sputum (Figure 1I). Likewise, there were no differences in the relative percentages of CD4+CD28+ and CD4+CD28− T cells (Figure 1F).
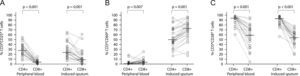
CD25 (panels A, D, and G, n=25), CD69 (panels B, E, and H, n=28), and CD28 (panels C, F and I, n=23) surface expression in peripheral blood and induced sputum T cells from patients with intermittent and moderate persistent asthma. The median value in each dataset is represented by a horizontal bar. Statistical analysis was performed with the Wilcoxon signed rank test.
The relative percentage of total T cells expressing CD25 and CD69 was significantly higher in sputum than in peripheral blood (26.9% vs. 21.3%; p=0.027 and 57.7% vs. 3.4%; p<0.001 for CD25 and CD69, respectively; Figures 1A and B). Similar results were obtained for CD8+ T cells (11.5% vs. 5.3%; p=0.001 and 74.4% vs. 4.8%; p<0.001, for CD25 and CD69, respectively; Figures 1G and (H). In contrast, for CD4+ T cells only CD69 expression was significantly higher in sputum when compared to peripheral blood (49.0% vs. 2.2%; p<0.001; Figure 1E).
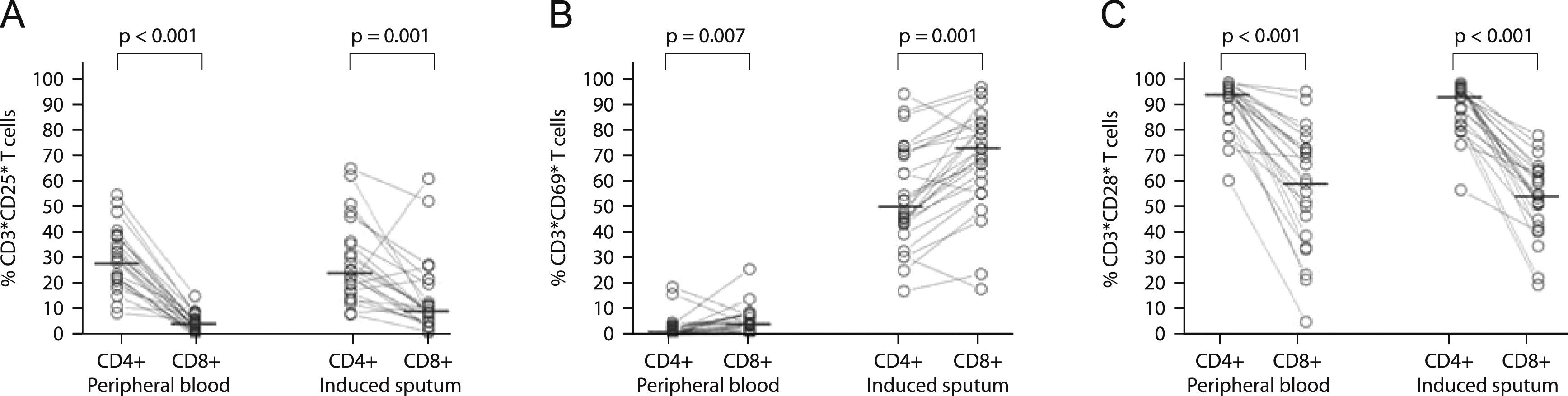
CD8+ T cells had a significantly lower expression of CD25 and CD28 than CD4+ T cells, both in induce=d sputum (10.4% vs. 25.6%; p=0.001, and 55.6% vs. 94.6%; p<0.001, for CD25 and CD28 respectively) and in peripheral blood (5.1% vs. 30.0%; p<0.001, and 61.7% vs. 95.9%; p=0.001, for CD25 and CD28 respectively; Figures 2A and C). In contrast, CD8+ T cells had a significantly higher expression of CD69 than CD4+ T cells both in sputum and peripheral blood (74.4% vs. 50.8%; p=0.001, and 4.8% vs. 2.2%; p=0.007, respectively; Figure 2B).
Comparison of surface expression of CD25 (panel A, n=25), CD69 (panel B, n=28) and CD28 (panel C, n=23), between CD4+ and CD8+ T cells from peripheral blood and induced sputum of patients with intermittent and moderate persistent asthma. The median value in each dataset is represented by a horizontal bar. Statistical analysis was performed with the Wilcoxon signed rank test.
To circumvent the possible inhibitory effect of inhaled corticosteroids on lymphocyte surface markers, we analysed the data referring only to intermittent asthmatics without medication, both at the time of sputum induction, and in the previous 2 weeks. We found results in these intermittent patients to be similar to those reported in the whole study population for the expression of activation markers in blood and induced sputum (data not shown).
Relationship between severity and phenotype in sputumAsthma severity was classified according to GINA guidelines and only intermittent or moderate persistent asthmatics were recruited for this study. Since we were unable to pair uncontrolled intermittent and moderate persistent asthmatic patients for analysis, we show here results for controlled intermittent and controlled moderate persistent asthmatics alone.
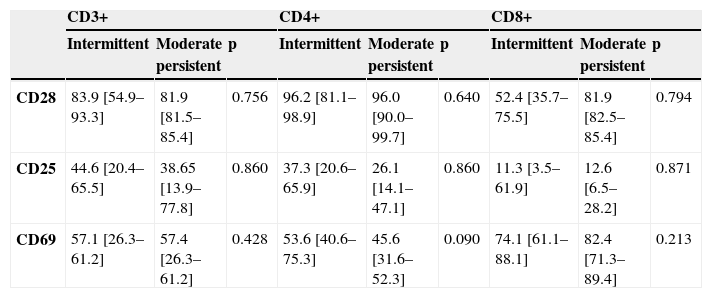
There were no significant differences in the percentages of any of the sputum T cell subpopulations between patients with controlled intermittent asthma and patients with controlled moderate asthma. Likewise, no association was found between relative expression in sputum lymphocytes of CD25 or CD69 and the severity of asthma (Table 3).
Expression of CD25, CD69 and CD28 in sputum total lymphocytes, CD4+ and CD8+ T cells from patients with intermittent (n=11) and moderate persistent (n=6) controlled asthma.
| CD3+ | CD4+ | CD8+ | |||||||
| Intermittent | Moderate persistent | p | Intermittent | Moderate persistent | p | Intermittent | Moderate persistent | p | |
| CD28 | 83.9 [54.9–93.3] | 81.9 [81.5–85.4] | 0.756 | 96.2 [81.1–98.9] | 96.0 [90.0–99.7] | 0.640 | 52.4 [35.7–75.5] | 81.9 [82.5–85.4] | 0.794 |
| CD25 | 44.6 [20.4–65.5] | 38.65 [13.9–77.8] | 0.860 | 37.3 [20.6–65.9] | 26.1 [14.1–47.1] | 0.860 | 11.3 [3.5–61.9] | 12.6 [6.5–28.2] | 0.871 |
| CD69 | 57.1 [26.3–61.2] | 57.4 [26.3–61.2] | 0.428 | 53.6 [40.6–75.3] | 45.6 [31.6–52.3] | 0.090 | 74.1 [61.1–88.1] | 82.4 [71.3–89.4] | 0.213 |
Results are represented as median percentage and range, and comparisons were performed by Mann-Witney U test.
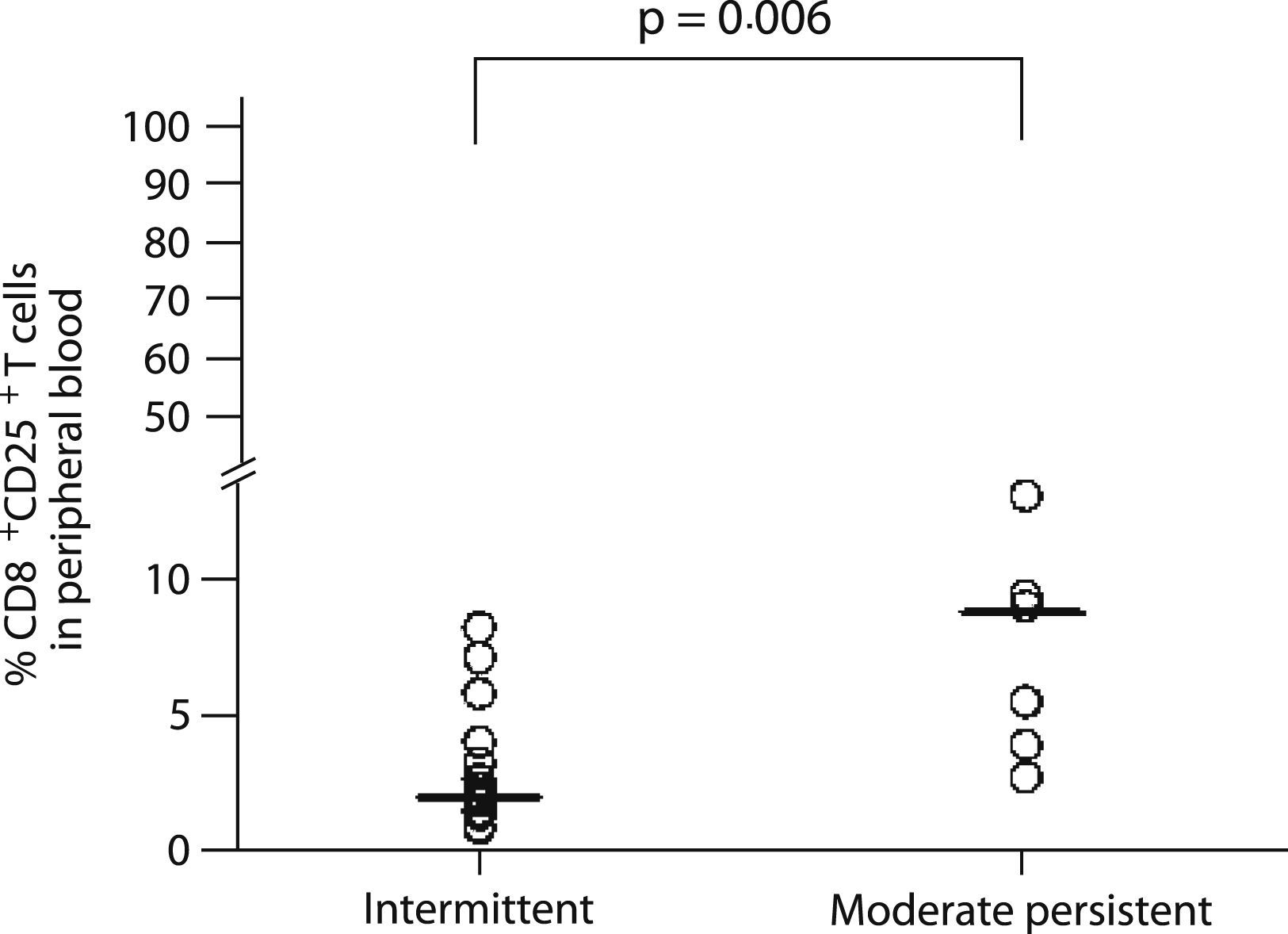
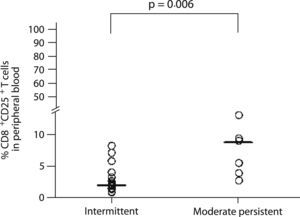
Nevertheless, CD25 expression on CD8+ T cells from peripheral blood was associated with disease severity, with moderate persistent asthmatics having a higher percentage of CD25 positive cells (8.7% vs. 2.1%, p=0.006; Figure 3) than the intermittent asthmatics.
Relationship between control and phenotype in sputumAsthma control was assessed prior to sputum induction by the ACT test.
Asthmatic patients were divided according to test results into controlled (ACT above or equal to 20 points) or uncontrolled (ACT below 20 points).
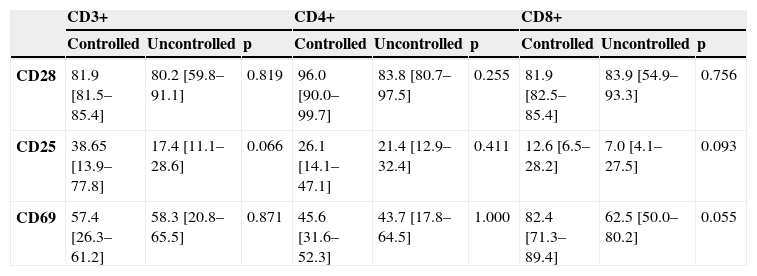
No association was found between relative expression in sputum lymphocytes of CD28, CD25 or CD69, and the control of asthma in moderate persistent asthmatics (Table 4).
Expression of CD25, CD69 and CD28 in sputum total lymphocytes, CD4+ and CD8+ T cells from patients with controlled (n=6) and uncontrolled (n=6) moderate persistent asthma.
| CD3+ | CD4+ | CD8+ | |||||||
| Controlled | Uncontrolled | p | Controlled | Uncontrolled | p | Controlled | Uncontrolled | p | |
| CD28 | 81.9 [81.5–85.4] | 80.2 [59.8–91.1] | 0.819 | 96.0 [90.0–99.7] | 83.8 [80.7–97.5] | 0.255 | 81.9 [82.5–85.4] | 83.9 [54.9–93.3] | 0.756 |
| CD25 | 38.65 [13.9–77.8] | 17.4 [11.1–28.6] | 0.066 | 26.1 [14.1–47.1] | 21.4 [12.9–32.4] | 0.411 | 12.6 [6.5–28.2] | 7.0 [4.1–27.5] | 0.093 |
| CD69 | 57.4 [26.3–61.2] | 58.3 [20.8–65.5] | 0.871 | 45.6 [31.6–52.3] | 43.7 [17.8–64.5] | 1.000 | 82.4 [71.3–89.4] | 62.5 [50.0–80.2] | 0.055 |
Results are represented as median percentage and range, and comparisons were performed by Mann-Witney U test.
In the present study, we observed higher expression of activation markers on sputum lymphocytes than on those in peripheral blood, consistent with an inflammatory process taking place in the lung. Interestingly enough, this heightened expression was predominantly significant in CD8+ T cells, which had higher expression of CD25 and CD69 activation markers. Only the percentage of CD69+ CD8+ T lymphocytes in the sputum showed a trend for association with asthma severity or control.
We found a higher CD4/CD8 ratio in induced sputum when compared to peripheral blood. Similar results have been previously reported by other investigators in asthma,3 and even in healthy volunteers.5 The CD4 : CD8 imbalance observed in our study was due primarily to a reduced percentage of CD8+ T cells. This may be due to the fact that CD8+ T cells may compartmentalise more intensely than CD4+ T cells within the submucosa rather than in the mucosa. However, in contrast, other researchers have found a tendency towards a high percentage of CD8+ T cells in the BAL of asthmatic patients as compared with healthy controls, resulting in a decreased CD4/CD8 ratio.18 In fact, it has been shown that CD8+ T cells are more sequestered than CD4+ T cells in the airway during an acute asthma attack.18 Furthermore, the number of CD8+ cells in bronchial biopsies in patients with asthma has been associated with disease outcomes, as determined by loss of lung function.19 Indeed, activated CD8+ T cell infiltration in peribronchial tissue has been associated with asthma death.10 Discrepancies between these results and ours may be due to the fact that different populations of asthmatic patients were studied, namely in terms of disease severity.
In the present study we did not find any significant differences between the percentages of either CD28-expressing CD4+ or CD8+ lymphocytes in sputum and peripheral blood. Although the low numbers of volunteers may account for the observed lack of differences, it should be borne in mind that effector T cells are less dependent on CD28 and their survival relies on extrinsic factors, such as IL-2, which are produced during activation.20 This may suggest that T cells found in the lung in the context of asthma are not different from those in peripheral blood, in terms of co-stimulatory requirements.
We also show here that in both total CD3+ cells and CD8+ T cells, the activation markers CD25 and CD69 were expressed in a higher relative percentage in sputum than in peripheral blood. Leckie et al. reported similar results to ours: both in asthmatics and healthy controls, CD8+ T cells from sputum had higher percentage of CD69 expression than CD4+ cells. There were also higher percentages of both CD4+ and CD8+ cells expressing CD69 in sputum than in peripheral blood.5 However, these authors failed to stain T cells with anti-CD3 mAb. Instead, they used anti-human CD45 mAb to exclude other non-leukocyte events. This might lead to the inclusion of CD8+ NK cells in the analysis. Moreover, the Leckie study only included eight “very mild” asthmatics, while in the present study, we performed comparisons based on severity between 11 subjects with intermittent asthma and 6 subjects with moderate persistent asthma, as well as comparisons based on control for 6 subjects with controlled, and 6 subjects with uncontrolled asthma. In another report, Ortega et al. assessed the presence of activated bronchial lymphocytes in induced sputum of asthmatic patients and healthy controls. Lymphocytes from asthmatics showed an increased surface expression of activation markers (CD25 in T cells, and CD23 in B cells) when compared to lymphocytes of healthy non-atopic subjects.21 However, this study only evaluated total T cells, while in our study we analysed both CD4+ and CD8+ subpopulations. Therefore, using a more detailed methodological approach, our study confirms and expands previous results observed by others. In our case, we could not compare our results with those obtained from healthy controls since only in two cases out of twelve were we able to analyse T lymphocytes. This suggests that only a minor fraction of healthy volunteers from our population, in the absence of any pro-inflammatory stimulus, may have significant numbers of lymphocytes in the airways.
Our previous results with phenotypic markers could have been influenced by sputum processing using DTT. However, we believe that this is not the case because adding the same concentration of DTT, as used in sputum processing protocol, to whole blood from six allergic asthmatic patients did not influence the expression of the markers under study. Furthermore, other studies have also shown that DTT has no effect on the expression of CD69 and other surface markers.5,22
We could not observe any relationship between asthma severity and the percentage of total CD4+ or CD8+ T cells expressing the activation markers CD25 or CD69 in induced sputum. A highly plausible explanation for not finding any difference in surface marker expression is the small number of participants in this study. In contrast, in peripheral blood, CD25 expression was increased on CD8+ T cells from patients with moderate persistent asthma as compared with patients with intermittent asthma.
We did not observe any association between of CD28 on sputum T lymphocytes and asthma severity, In this regard, our results are not in agreement with those from a previous report. Hamzaoui et al. studied, for the first time, the expression of CD28 on CD8+ cells in induced sputum. CD8+CD28− cells were found to be more expanded and expressed lower levels of IFN-γ in severe asthmatics than in mild asthmatics and age-matched healthy controls.23 However, the authors did not include CD3 in the flow cytometric evaluation, including as CD8+ T cells probably other non-T cells (namely NK cells) which may also express CD8.
Reasons that may account for our failure to observe a relationship between asthma severity and the biological parameters analysed in the sputum in our study may include the fact that we studied low numbers of patients. Another reason may be our patients with moderate asthma were on inhaled corticosteroids. In fact, treatment with inhaled corticosteroids could have had a confounding effect on analysis of airway inflammation, as it has been demonstrated to reduce expression of some activation markers.24 Alternatively, disease severity may rather be related to changes in function than the percentage or phenotype of CD8+ T cells in the bronchi of patients with asthma.
Independently of disease severity, it is important to monitor control of disease. In our study, no significant association was found between relative expression of CD28, CD25 or CD69 on sputum lymphocytes and the control of asthma. However, it should be stressed that expression of CD69 on sputum CD8+ T lymphocytes showed a trend towards being significantly increased in moderately severe asthma, when controlled patients were compared with uncontrolled patients (82.4 (71.3–89.4) vs 62.5 (50.0–80.2); p=0.055). This may suggest that activated CD8+ T lymphocytes with a regulatory role (but not of the CD28- subtype) or with a Tc1 type of cytokine pattern may be associated with better asthma control. In this sense, it has been shown that CD8+ T cells are important immunoregulatory cells in some animal models of respiratory allergic disease.25 This possibility should be addressed in a subsequent study involving a larger number of patients. In fact, it is possible that the lack of significant association between asthma control and phenotypic activation markers or CD28 may be due to the fact that we included few patients or that all moderate persistent asthmatics were taking inhaled corticosteroids. Studies with a different design, allowing suspension of medication may also help to clarify this issue.
In summary, in this study we show that CD4+ and CD8+ T cells are activated in the lung, which may suggest that both subpopulations are participants in the inflammation process at the target organ. However, we have not been able to demonstrate a relationship between activation of T cell subsets and severity of asthma or asthma control, except for a trend for a higher percentage of CD69+ CD8+ T lymphocytes in the sputum to be associated with control. Further studies are needed in order to characterise the inflammatory and regulatory function of T cells in the lung, mainly the CD8+ subpopulation.
Conflict of interest statementProf. Olga Lourenço has no conflicts of interest to disclose.
Prof. Mafalda Fonseca has no conflicts of interest to disclose.
Prof. Luis Taborda-Barata has received honoraria from AstraZeneca and Merck.