INTRODUCTION
The prevalence of atopic disease has been increasing in many parts of the world over the last 20-30 years1-4. "Westernized" life style has been found to be associated with this trend5. The exact reasons for the increasing prevalence of atopy are not known. Yet, increased allergen load (e.g. mites)6, exposure to tobacco smoke7 and air pollution by automobile exhaust8 seems to be the suggested causes. Recently; the preventive effect of early childhood infections on allergic sensitization has been the mainstay of many studies9. Findings of an inverse relationship between occurrence of atopy and number of siblings10,11, findings of a very high prevalence of asthma in certain islands with low prevalence of respiratory infections12 and the findings of an inverse association between past measles infection13 and atopy; and past hepatitis A infection and atopy14 has been supporting this hypothesis. The reason for the suggested inhibitory effect of infections on the development of atopy could be that the infections by inducing Th-1 cytokines, direct the immune system to this branch of the T helper cell immune response15. Interferon-gamma, which is an important cytokine of the Th-1 response, can suppress the Th-2 responses characteristic of atopy16.
Mycobacteria, especially M. tuberculosis, are known to be potent inducers of Th-1 immune response17. In tuberculosis, an important marker of Th-1 mediated acquired immunity is the development of delayed type hypersensitivity. This can be tested by observing the reaction after 48 hours, to the intradermal injection of tuberculin protein. Recently Shirakawa et al. reported a strong inverse association between delayed hypersensitivity to M. tuberculosis and atopy in Japanese children18. Similarly, Strannegard et al. had investigated this relationship in Swedish children but could not find an association between BCG vaccination and atopy19.
From the point of view that some local factors could have been effecting this association, we aimed to investigate the relationship between M. tuberculosis infection and atopy in children living in Istanbul, Turkey.
MATERIAL AND METHODS
The study population consisted of 252 children. All the patients were fulfilling the "American Thoracic Society" criteria for asthma20. All the patients had been BCG vaccinated in the newborn period. After the demographic data (age and gender) were recorded, PPD (Purified Protein Derivative of Tuberculin) testing was performed to all patients. The testing was performed on the volar face of the forearm by five tuberculin units. Seventy-two hours later the induration diameter was measured by "ball-point pen" method. Patients having induration less than five millimeters were accepted as PPD negative. Patients having induration diameter more than five were accepted as PPD positive. Patients having PPD induration of more than 10 mm in diameter were further investigated with thorax HRCT and erythrocyte sedimentation rate for a possible tuberculosis disease. Later on, allergy skin testing was performed to all patients by using "Quintest multitest applicator" (Hollister-Stier). The most common aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Alternaria, Aspergillus fumigatus, cat and dog dander, feather, grass, common trees - Stallergenes/France) were tested in this respect. The testing was performed on the volar face of the forearm. Children having wheal diameter more than three millimeters to at least one of the most common aeroallergens were accepted as atopy positive.
Statistical analysis was performed by using Graphpad Instat 3.05. The PPD (+) and PPD (-) groups were compared with respect to their ages by using Student t test. They were compared with respect to their gender and allergy skin prick test positivity rate by using Fisher's Exact test.
RESULTS
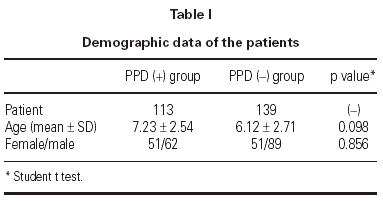
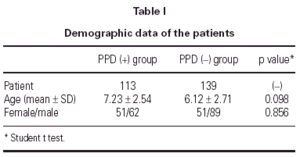
In 139 children delayed type hypersensitivity reaction to Mycobacterium tuberculosis was negative (< 5 mm). This group was named as PPD (-). The mean age in this group was 6.12 (2.71 years. There were 89 boys and 51 girls (table I). In 113 patients the PPD reaction was positive (> 5 mm). This group was named as PPD (+). In PPD (+) group the mean age was 7.23 ± 2.54 years. There were 62 boys, 51 girls (table I).
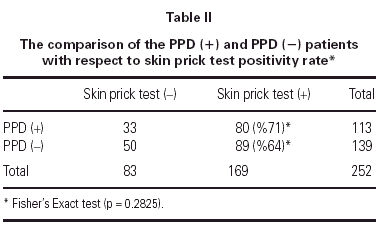
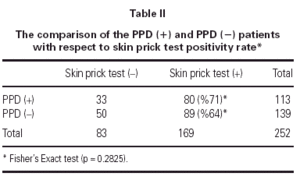
In PPD (-) group 64 % (n = 89) of the patients had a positive reaction to at least one common aeroallergen, in PPD (+) group 71 % of the patients had positive reaction to at least one common aeroallergen. There were no statistically significant differences between the two groups with respect to their ages and gender (p = 0.098 and p = 0.856, respectively). As the two groups were compared with respect to their skin prick test positivity rate, no statistically significant difference was detected between them (p = 0.283) (table II).
In the PPD (+) patients, the ones with a induration diameter > or = 10 mm were further investigated for a possible tuberculosis disease with thorax high resolution computerized tomography and erythrocyte sedimentation rate. Non of the patients had tuberculosis disease.
DISCUSSION
In this study no statistically significant difference was detected between Mycobacterium tuberculosis infection and allergy development.
In recent decades there has been an increase in severity and in prevalence of atopic disorders in developed countries. Studies on migrants from developing to developed countries support the importance of etiological environmental changes associated with "Westernization". One factor temporarily associated with the rise of atopy is the decline of many infectious diseases in developed countries as a result of improved living standards and immunization programs18. The hypothesis that infections may inhibit development of atopy postulates that infectious agents, such as viruses and intracellular bacteria, preferentially stimulate the development of Th-1- type immunity. Therefore a relative lack of infections caused by such agents during early childhood might lead to a shift of the Th-1/Th-2 balance to the Th-2 side, thereby predisposing for allergic sensitization and development of atopy. In this context, Mycobacteria, which are strong inducers of a Th-1-type immune response are particularly relevant19.
The first study that investigated the association between Mycobacterium tuberculosis infection and atopy was reported by Shirakawa et al. in 199718. The investigators had searched for serum specific IgE positivity rate in PPD (+) and PPD (-) children and had found that PPD (+) patients has significantly less specific IgE positivity rate. In our study we investigated atopic status by intradermal skin prick tests which is accepted to be more sensitive than serum specific IgE measurements. This might be one of the reasons for the different results seen in the two studies.
Similar to our results, Strannegard et al. who had investigated the atopic status in BCG vaccinated and unvaccinated children in Sweden, report that they had found no statistically significant difference between the two groups with respect to having atopy. Although not statistically significant they report that BCG vaccinated children had lower frequency of allergy than unvaccinated children. They also indicate that these were immigrant children who had been born in another country not in Sweden. They suggest that some factors, other than BCG vaccination, attributable to the environment in the children's country of origin, could have protected against development of allergy19. Again in agreement with our results, Alm et al. report no correlation between BCG vaccination and absence of atopy in a case control study of Swedish children21.
Mycobacterium vaccae, a saprophyte mycobacterium found in the environment, is a strong stimulant of Th-1 type immune response. Experimental studies with this Mycobacteria shows that it is effective in decreasing serum levels of IgE and IL-5. Tukenmez et al. who had investigated the possible anti-allergic effects of Mycobacterium vaccae on Balb-C mice report that M. vaccae had to act synergistically with M. Tuberculosis in order to induce Th-1 type immune response, thus inhibit atopy22.
The level of saprophyte Mycobacteria in the local environment of a country might be important in inhibiting the atopic status in children who had past Mycobacterium tuberculosis infection. This might explain different results in different countries with respect to M. tuberculosis infection and atopy association.
We conclude that, future studies investigating the levels of saprophyte Mycobacteria in different localizations and their synergistic relations with Mycobacterium tuberculosis should reflect more light on this hypothesis.