INTRODUCTION
Airway inflammation in asthma is the primary event that leads to the airways obstruction and hyperresponsiveness. Induced sputum (IS) has been used in this last years as a reliable technique to investigate the airway inflammatory process non-invasively (1). It has been considered a highly reproducible method without significant adverse effects and with comparable results to those obtained with other more invasive techniques such as the bronchoalveolar lavage or the bronchial biopsy (2). To increase the effectiveness of this method, the fluorocytometric analysis of induced sputum has been recently introduced, providing useful information about components of the inflammatory response, quantifying cells and describing different inflammatory markers.
However, on the sputum, this technique has to face different methodological difficulties. The viscosity of the sample to analyse, requires a previous processing, which implies the use of a mucolytic agent to improve cell dispersion. The reducing mucolytic agent used most often, has been dithiothreitol (DTT), which allows an appropriate homogenization of the sputum sample. It has been previously described that DTT does not interfere over cellular counts although it could alter the detection of some other cellular markers (3, 4).
The primary aim of this study was to further examine if DTT could affect the identification of some phenotypic characteristics of different cell populations involved in the inflammatory process. Among the adhesion molecules, Very Late Activation Antigen-4 (VLA-4) is an α4 β1 integrin, a cell surface heterodimer expressed on leucocytes. It is found in all mononuclear leucocytes, eosinophils and basophiles but it is absent from neutrophils (5). VLA-4 mediates cell-cell adhesion to the Vascular Cell Adhesion Molecule-1 (VCAM-1) and cell-matrix adhesion through the binding to fibronectin. Nevertheless, there is still some controversy about the expression of VLA-4 on eosinophils in induced sputum of asthmatic patients. Bocchino et al, reported intracellular but no surface detection of VLA-4 in IS cells with an immunohystochemical procedure (6). On the other hand, Diwen Quieu et al. suggested a decreased level of the VLA-4 surface expression on eosinophils in blood by flow cytometry measurement proportionally to the used DTT concentration (7). Both investigators measured VLA-4 levels using anti CD49d monoclonal antibody (CD49d represents the α4chain of the whole heterodimer molecule, being CD29 monoclonal antibody the β1 chain).
We have tried to validate the effectiveness of flow cytometry in induced sputum analysis to assess airway inflammation in asthma. We could consider that it is a reliable technique although DTT could interfere over the detectable levels of adhesion molecules on eosinophils and lymphocytes as DTT affects the VLA-4 cell detection especially on eosinophils.
METHODS
Subjects
Sputum was induced in June, 2000 from 7 non- smoker subjects, aged 19-34, 4 patients with seasonal bronchial asthma and 3 healthy non-atopic subjects, without an upper respiratory tract infection in the previous 8 weeks. Asthma was defined as a clinical history of cough, dyspnea, or intermittent wheeze during the grass pollen season in Madrid (May and June), with documented reversible airflow limitation (an improvement in FEV1 of > 12 % after inhaled salbutamol) and an FEV1 > 70 % predicted. Atopy was indicated by a positive skin-prick test (> 3mm wheal) to grass pollen extract (ALK-Abelló. Madrid. Spain). They only used inhaled chromoglycate or less than 800 μg per day of budesonide to control the asthmatic disease. For 15 days before the moment of the test, they were allowed to use exclusively short acting bronchodilator taken as required.
Healthy subjects had no history of any respiratory symptoms, had an FEV1 > 80 % predicted with no significant changes after Salbutamol (200μg) and no methacholine airways responsiveness after the inhalation of > 16 mg/ml.
All subjects signed consent forms. The protocol was approved by the ethical scientific committee.
Sputum induction and processing
The sputum was induced and processed according to Pizzichini's method with some modifications (8), by the inhalation of 3 % hypertonic saline nebulized. Saline solutions were nebulized by an ultrasonic nebulizer (Ultra-NEB-2000, De Vilbiss). The collected sputum samples were examined within 2 hours.
To avoid as much as possible the presence of salivary contamination in the sputum sample, selected portions from the whole expectorate were taken using an inverted microscope. Osmolarity was corrected by adding four times the weight of the sputum of PBS buffer solution. DTT 0.1 M (Sigma Co. Texas. USA)was added reaching a 3 μM final DTT solution. Unfortunately, the management of no DTT-treated sputum was not possible because of the viscosity of the samples.
After agitating in a vortex mixer for 15 seconds, the samples were placed on a shaking plate for 30 min., room temperature. The suspensions were filtered through a 30 μfilter (Miltenyi Biotec S.L. Madrid. Spain) and were spinned at 1400 r.p.m. for 7 min. The supernatants were stored at 40 °C for later analysis. The pelleted cells were resuspended in PBS. Total cell counts and viability (Trypan Blue exclusion method) were determined.
Cytometry
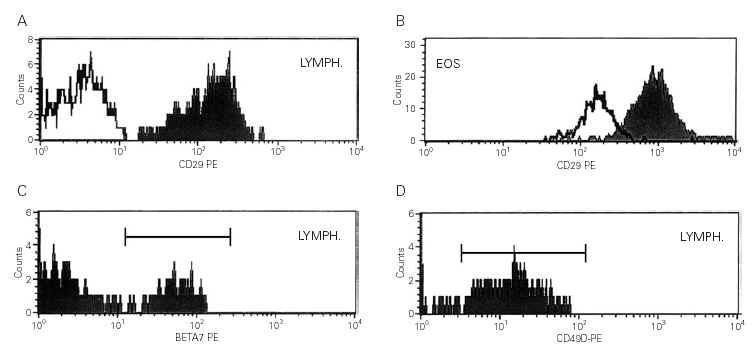
For each measurement, 100.0000 cells were incubated in the dark with a combination of 3 fluorocrome-conjugated monoclonal antibodies(mAb) (Becton Dickinson Immunocytometry Systems,San Jose, CA,USA) (table I) for 30 min. at 4 °C.
After centrifugation, the supernatant was discarded and the pellet resuspended in 0,9 % saline solution. A FACScan flow cytometer (Becton Dickinson) was used for the cell acquisition and to measure the mean fluorescent intensity (MFI). Cell Quest Software (Becton Dickinson) was used for the analysis. All markers were compared with the background of conjugated isotypic-control mAb.
Intracellular staining was done in 3 samples after fixation and permeation with Cytofix-Cytoperm (Pharmingen, Los Angeles, CA., USA). For the whole blood analysis, FACS Lysing solution (Becton Dickinson) was added to each sample for hemolyzation of red blood cells and the final pellet was also incubated in the dark with the same combination of 3 fluorocrome-conjugated mAbs. Cell-Quest Software (Becton Dickinson) was also used for analysis.
RESULTS
Sputum induction was performed successfully in all subjects without any salbutamol requirement after the induction.
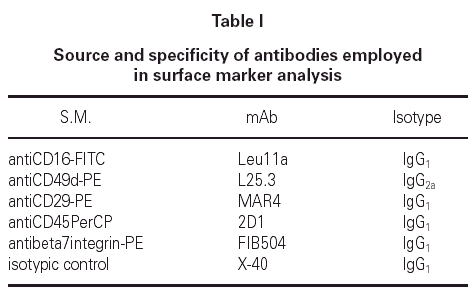
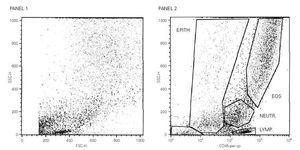
A total cell count of 1.800.000 + / 200.000 mL1 was obtained in all the samples and there was no significant squamous cell contamination in any of them. The analysis windows for lymphocytes and eosinophils were established on the basis of their levels of granularity/complexity (side scatter) and level of expression of CD45 (fig. 1). In all the subjects, asthmatics and non-asthmatics, similar cell counts values were found (not shown).
Figure 1.--Panel 1. Biparametric representation of size (forward scatter, FSC) against granularity (side scatter, SSC) in induced sputum cells. Panel 2. The expression of CD45 allows the identification and quantification of eosinophils (upper gate) and lymphocytes (lowest gate).
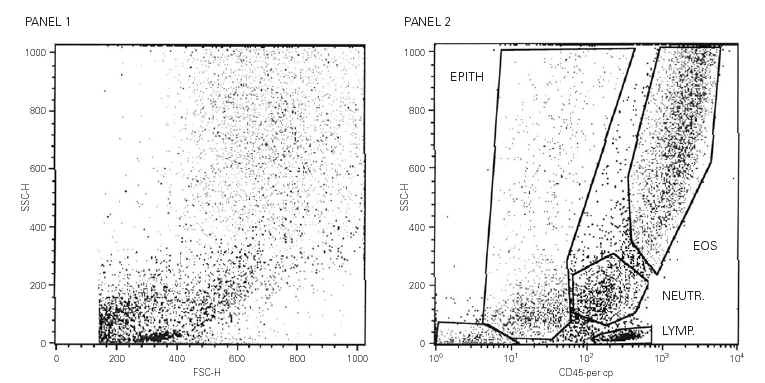
CD49d detection levels in eosinophils were much lower than expected, affecting both the MFI and the stained cells percentage. Not more than 7 % of the eosinophils exhibited CD49d in their surfaces in any of the subjects and no intracellular detection was either observed (not shown). CD49d could be detected on peripheral blood eosinophils and lymphocytes but the intensity of the staining was reduced in more than 30 % after the blood sample was incubated in 3 μM DTT. This DTT effect was even more intense on eosinophils where a drop of 50 % in the MFI value was observed (fig. 2).
Figure 2.--In Panel A, representative flow cytometric analysis histograms of the detection of CD49d on peripheral blood eosinophils. The filled dark grey histogram corresponds to the absence of DTT treatment. The open dark grey histogram shows the detection after exposure to DTT. The fluorescence of the isotype control is represented in light grey. In Panel B, the filled grey histogram stands for the detection of CD 49d on no DTT-treated blood lymphocytes. The open grey histogram represents DTT-treated blood lymphocytes. The fluorescence of the isotype control is very low and overlaps with the Y-axis.
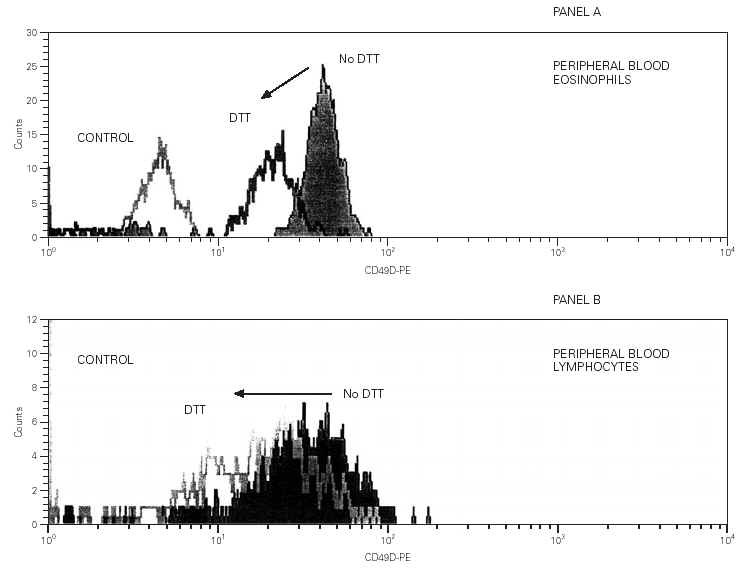
No DTT effect over CD16 and CD45 detection levels was found on both cellular populations, lymphocytes and eosinophils. No DTT effect was either observed in β7 on lymphocytes and CD29 on both cell populations (fig. 3).
Figure 3.--Panel A. The filled histogram represents the detection of CD29 on IS lymphocytes. The open histogram corresponds to the isotype control fluorescence. Panel B. The filled histogram represents the detection of CD29 on IS eosinophils. The open histogram corresponds to the isotype control fluorescence. Panel C. The filled histogram represents the detection of β7-chain on IS lymphocytes. The open histogram corresponds to the isotype control fluorescence. Panel D. The filled histogram represents the detection of CD49d on IS lymphocytes. The isotype control fluorescence is on the Y-axis.
On the other hand, although the detection of CD49d in the surface of lymphocytes in induced sputum was also altered, it seemed to be lower than in eosinophils (fig. 3).
DISCUSSION
Asthma is a chronic disease characterised by ongoing inflammation in the airways. Physicians regularly use symptoms and lung function tests as main tools in diagnosis and monitorization of the disease evolution. Although these data may offer principal information, we should not forget the airways inflammation examination. In order to provide complementary information in this field, induced sputum has been shown as a non invasive alternative to bronchoalveolar lavage and bronchoscopy (2). This study shows that sputum can be induced safely in mild asthmatic patients. In all these 7 subjects, asthmatic and non-asthmatics no significant drops in FEV1 values were found after the induction procedure with a 200 μg inhaled salbutamol pre-treatment.
Flurocytometric analysis of induced sputum has been rarely used to determine the phenotypic characteristics of lymphocytes in asthmatics since Kidney et al (9) in 1996. They selected a group of smokers as controls because it was considered that a non-smoker healthy subject would be unable to expectorate enough sample to be analysed by flow-cytometry. Interestingly, sputum was induced successfully in all these cases, avoiding the effect of smoking over the adhesion molecules cell expression. Moreover, no differences in total cell counts between asthmatics and healthy subjects were found. We believe these findings are related with an stable situation of the disease in the moment of testing as it was described in samples obtained from teenagers with mild stable asthma and not regular treatment requirements (10).
On the other hand, it has been repeatedly reported the presence of higher percentages of inflammatory cells expressing adhesion molecules in asthmatic patients (11, 12). However, we have been unable to detect intracellular or surface expression of CD49d on eosinophils in asthmatics and healthy subjects. Moreover, we found a lower level on lymphocytes than expected. We believe our findings reflect the effect of the DTT over the staining and not a real decreased level of the markers in close agreement with Diwen Quiu's findings in blood analysis.
How DTT alters CD49d detection is unknown. DTT is able to destroy epitopes, modifying the protein structure by opening disulphide bonds and probably changing the α-chain configuration because no drops in CD29 (VLA-4/β -chain) detection have been observed. Besides, the recognition of other adhesion molecules (CD54-ICAM-1 or CD40)seems not altered by DTT (data not published yet).
The differences between CD49d surface detection in eosinophils and lymphocytes might be explained by two different factors. We have observed that IS lymphocytes express b7-chain protein. This molecule constitutes part of the mucosal homing heterodimer α4β7, which is not detected on eosinophils. We have found CD49d on lymphocytes that could correspond to the a4 presented in the α4β7 heterodimer instead of the VLA-4 heterodimer α4β1. On the other hand, the effect of DTT on CD49d on peripheral blood lymphocytes seemed to be less intense than the effect over CD49d on peripheral blood eosinophils (fig. 2) which might be related with conformational differences or merely higher initial density of VLA-4 on lymphocytes.
In conclusion, IS has been shown as a safety, successfully tool being fluorocytometric analysis a good complementary technique in the investigation of airways inflammation. However, the effect of DTT treatment could alter the recognition some adhesion molecules on inflammatory cells, clearly interfering with CD49d detection levels.
ACKNOWLEDGEMENTS
We thank Luis Zayas, Maria Angeles Castillo and Francisca Casco for technical assistance.
This study has been supported by a grant from the Allergy and Clinical Immunology Spanish Society Foundation.