INTRODUCTION
Inhaled corticosteroids are increasingly used as maintenance treatment in childhood asthma 1. While inhaled corticosteroids are safer than oral corticosteroids many physicians are concerned about the potential side effects. It is still unclear whether the long term inhaled steroid usage has negative effects on growth, bone mineral density and adrenal function and there are some suspicions and worrying about its safety 2-5. There are several reports about different side effects of inhaled steroids with different dosage and duration 2-10. However most of the studies focused on particularly linear growth, adrenal suppression and bone density. Results on these side effects during corticosteroid therapy are conflicting. Several studies have reported poor correlation between corticosteroid-induced short term changes in the growth rate 2,3,10-14. On the other hand completely normal growth has been reported in children receiving ICS therapy 13,15-18. The reports on the effects of ICS one bone mineral density have been controversial, some showing a reduction in BMD in relation to ICS use whilst others have not 19-26. Adrenal suppression is another important point to discuss in children treated with ICS.Some investigators have found mild to moderate suppression of adrenal function while others have normal adrenal function 11,24-34.
In this randomised study we aimed to investigate the effect of long term inhaled steroids on linear growth, bone mineral density and adrenal glands in asthmatic children.
PATIENTS AND METHOD
Thirteen children with moderate asthma that were on follow up by Cukurova University, Pediatric Allergy-Immunology Division were enrolled to the study. Asthma was diagnosed according to guidelines for the diagnosis and management of asthma position paper 1. The study was conducted between January 1999 and September 2000. Patients were randomly divided into two groups. Fifteen children treated with inhaled 400 mg/day budesonide (Group I) and 15 were treated with 250 mg/day fluticasone propionate (Group II). Control group included 30 children (Group III).
Anthropometric assessments (weight, height, body mass index), pulmonary functions including vital capacity, peak expiratory flow rate (PEF),forced expiratory volume in the first second of expiration (FEV1), symptom and medication scores 35 were evaluated at the beginning of the study and after a one year period.
Bone metabolism
Serum calcium, phosphorus, Alkaline Phosphates levels, urine calcium and creatinine in 24 hours collected urine were measured and urine calcium/creatinine ratio was calculated in the beginning and at the end of the study.
Bone mineral density was measured at L2-L4 vertebra by using dual energy X-Ray absorbtiometry (DEXA) method before and after therapy.
Adrenal functions
Basal morning serum cortisol level, free cortisol in 24 hour urine collection, urine cortisol/creatinine ratio was evaluated in the beginning and at the end of the study. ACTH stimulation test by using synacten injection was performed before and after the study.
STATISTIC
SPSS program (version 10.0) was used for all statistical analysis. Analysis was performed by Mann Whitney-U and Wilcoxon tests. A p value less than 0.05 was considered significant.
RESULTS
Group I (budesonide group) included 15 patients. There were 8 boys (%53.3), 7 girls (%46.7) with the mean age of 10.6 ± 2.1 years (range: 7-13 years). Group II (fluticasone propionate group) included 9 boys (%60), 6 girls (%40) with the mean age: 9.6 ± 2.6 years (6-12 years).
Control group contained 30 patients. Of the patients 18 were boys, 12 were girls with the mean age of 9.7 ± 1.7 years.
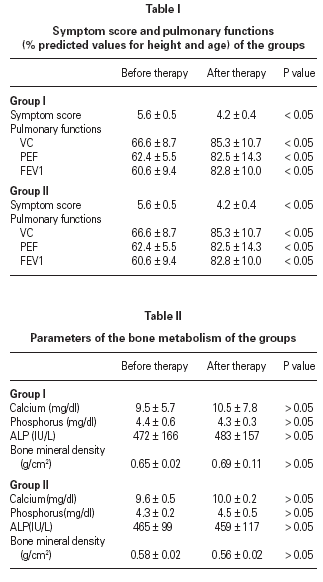
Symptom and medication scores and pulmonary functions, were improved significantly after the inhaled steroid treatment in both groups (table I)
Anthropometric measurements and linear growth
Body mass index and weight percentiles did not change after one year in all groups (p > 0.05). The mean increase in linear growth from the beginning to the first year of the therapy was statistically significant and similar in all groups (p < 0.05). Growth rate (cm in one year) was 8.4 ± 4.6 cm in group I, 8.2 ± 6.2 and 8.1 ± 5.1 cm in group II and control group, respectively.
Growth rate was found to be smaller than 4 cm in one patient in group I (3.2 cm), in one patient in group II (2.7 cm).
Bone metabolism
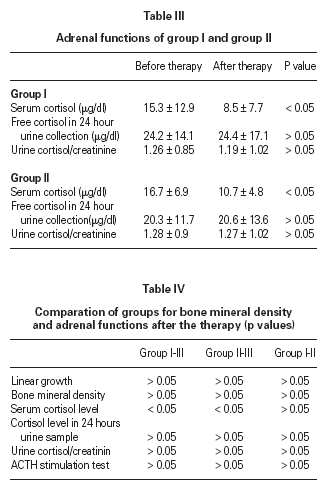
There were no significant differences in serum calcium, phosphorus and ALP levels between the baseline and end values (p > 0.05) in all groups. The bone mineral density of group 1 and group 2 at the beginning and in the first year was similar (p > 0.05) and comparable with the control group (p > 0.05) (table II).
Adrenal functions
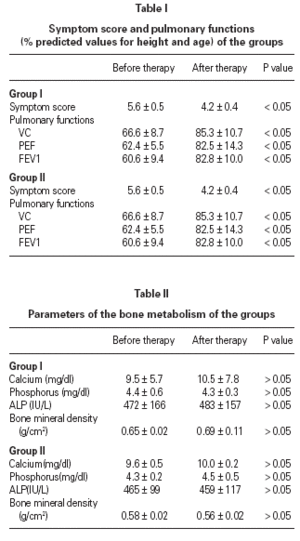
Mean basal serum cortisol level diminished at the end of the therapy but no significant differences were found between the initial and the end values in respect to the urine cortisole levels and cortisol/creatinin ratio in all groups (table III). Of three groups ACTH stimulation test revealed that there were no significant differences between group I, group II and control groups (table IV).
DISCUSSION
Inhaled corticosteroids are recommended as first line antiinflammatory therapy for the treatment of asthma. Many short and long term studies have shown ICS to be of benefit in children with asthma. However concern about the potential for systemic adverse events, including linear growth, bone metabolism, suppression of adrenal glands, has resulted in reluctance on the part of many physicians and parents to use ICS.
In this study we evaluated the long term effects of inhaled budesonide and fluticasone propionate and found that long term ICS treatment did not cause any serious side effects in children.
Linear growth
The reports on the effect of ICS have been contraversial. Both retarded growth and completely normal growth have been reported. It was concluded that there is no significant evidence of adverse effects on growth when conventional doses (< 400 mg/day) of ICS used 2,5,13,36.
Long term analysis of the effect of ICS on growth is available predominantly for beclametasone dipropionate. Studies have reported slowing of growth rates with beclametasone dipropionate when compared budesonide or fluticasone propionate 10,12,36.
In our study linear growth was found to be comparable in both budesonide and fluticasone propionate with control group. This finding supports the recent studies in which impaired growth was not noticed in asthmatic children receiving ICS in doses that have been considered safe. However in our study Growth retardation was detected in two patients (one in budesonide group, one in fluticasone propionate group) for the real effect of ICS on growth of asthmatic children, good information for their growth before the treatment with ICS should be available. Most studies suffered from a lack of baseline growth velocity data, baseline differences in age and height between treatment groups, and reliance on short term measurements which do not accurately predict long term growth 2. In recent years Agertoft et al reported that growth rates were significantly reduced during the first years of budesonide treatment, these changes in growth rate were not significantly associated with adult height 15. Children with asthma who received long-term ICS attain normal adult height 15-18. A weakness of our study is that we did not have any information about baseline growth of these children. On the other hand these children should be followed for adult height. These factors should be addressed in a well designed study of drug treatments on growth.
Bone metabolism
Long term use of oral corticosteroids is associated with decreased bone density and an increased risk of bone fracture 22. Inhaled corticosteroids have a considerably better safety profile than oral steroids. Recent studies with ICS have yielded contradictory results, with some showing a reduction in bone mineral density whilst others have not 3,4,19-26. We have not investigated negative effect on BMD neither with budesonide nor with fluticasone propionate. Reduction in BMD has not been reported with the dosage of 200-400 mg/day whereas subtle changes have sometimes been seen with high dose therapy. However, in recent year's negative effects of budesonide on bone turnover with the dosage of 400 mg/day has been reported 19,23,24,37.
We used DEXA for the evaluation of BMD. DEXA provides a measure of areal bone mineral density. And by measuring of volume of scanned bone, changes in the mineral density and bone size in growing children could be distinguished 20.
Studies of biochemical markers of bone metabolism have shown a reduction in serum osteocalcin concentration, whereas other markers such as ALP and procollogen peptide I have shown less consistent results 19,37-40. We did not found any change in biochemical markers such as serum calcium, phosphorus, ALP levels and calcium/creatinine ratio in 24 hours collected urine. However we could not have a chance to evaluate serum osteocalcin levels because a lack of measurements techniques.
Dietary calcium intake is another important point to discuss. Some authors think that decreased BMD may be partly related with low calcium intake. Maintenance of adequate calcium and vitamin D intake thought to be helpful for negative side effects of ICS on bone turnover 5,7,41. We could not be able to evaluate dietary calcium intake in this study, but we did not detected reduction in calcium levels and BMD.
Adrenal Functions
HPA-axis function is an important indicator of systemic exposure to corticosteroid therapy. Studies of the incidence of adrenal suppression in asthmatic children receiving ICS have revealed conflicting results 2,6,8,11,26-29. Mild to moderate HPA suppression, symptomatic adrenal insufficiency and normal adrenal function were reported in different studies 32,33. The results are conflicting mainly because the investigations have often been uncontrolled, patients have previously received oral steroids, the duration of therapy have varied, different inhalers have been used, and different tests have been applied to monitor changes in HPA-axis function.
Various tests have been used to evaluate HPA-axis function including measurement of basal morning plasma cortisol concentration, integrated plasma cortisol concentration over the course of > 12 to 24 hours, urinary free cortisol concentration and ACTH stimulation test. In this study we evaluated HPA-axis function by using measurement of basal morning plasma cortisol concentration, urinary free cortisol concentration and ACTH stimulation test. We found that basal morning plasma cortisol concentration was decreased after ICS treatment. Similar to our results lower levels of morning cortisol were found in asthmatic receiving budesonide, beclametasone propionate, and fluticasone propionate 4,6,11,27. The ACTH stimulation test and 24 hour integrated plasma cortisol concentration are methods recommended by the US Food and Drug Administration (FDA) to evaluate HPA-axis evaluation 34. No significantly changes were detected ACTH stimulation test in our study. Adrenal suppression could occur in children with the use of 400-1000 mg/day budesonide 2,11,32,33. In another report it was detected that fluticasone propionate exhibited at least two fold greater adrenal suppression than budesonide on a microgram equivalent basis 28.
Free cortisol in the urine (UFC) is frequently measured in clinical research to assess whether inhaled corticosteroids (ICS) cause suppression of the hypothalamic-pituitary-adrenal axis. We also evaluated urinary free cortisol concentration. However it was thought to be an unreliable surrogate marker of adrenal suppression 42.
We did not observe any serious effect on adrenal glands; we think that it may because we used conventional doses in our patients.
In conclusion we observed that long-term usage of inhaled steroids (250-400 mg/day) did not have any important side effects on linear growth, bone metabolism and adrenal functions, however we suggest that children treated with inhaled corticosteroids for a long time should be followed closely with respect to side effects.