INTRODUCTION
Aqueous allergenic extracts have been used at the beginning of the clinical use of Specific Immunotheraphy (SIT), but they have been gradually substituted by depot extracts. This latter kind of preparation was introduced in the forties and since then has gained large success in medical practice to treat IgE-mediated respiratory pathologies (1-3). Allergens adsorbed to aluminum hydroxide, tyrosine or calcium phosphate are slowly released from the injection site, leading to fewer side effects and simpler administration schedules.
Venom Immunotherapy (VIT) is still performed in most European countries almost exclusively with aqueous extracts, whereas depot extracts are the common choice in SIT for inhalant allergens. In German-speaking countries, on the contrary, the switch from aqueous to depot preparation has taken place also for VIT.
According to the experience done with inhalant allergens, a better tolerance and similar efficacy can be expected from depot as compared to aqueous preparations for VIT, efficacy being mainly linked to the maintenance dose of allergen administered (4).
A solid evidence in favor of VIT efficacy with venoms from Apis an Vespula performed with aqueous preparations is available (5-8), whereas only a few open trials showing safety and efficacy of VIT with depot extracts have been published (9-11) in spite of the large use of these preparations in some countries.
Because most patients included in these studies were sensitized to Apis (9, 11) or have been treated with aqueous preparations (10), we designed this retrospective study focussing on patients sensitized to Vespula to improve the evidence already existing about depot preparations in such patients.
MATERIAL AND METHODS
Selection of patients
Patients were retrospectively included in this study if admitted to VIT fulfilling the following criteria:
Clinical history of hypersensitivity to Vespula spp. with identification of the responsible hymenopter.
Systemic reactions of Grade III-IV according to Mueller (12) after a Vespula spp. sting.
High risk of new exposure to the allergen for patients with systemic reaction Grade III (professional firemen and ice-cream vendors, amateur fishermen or farmers, etc.).
RAST at least class 2 for sera IgE specific to Vespula spp. venom.
Venom immunotherapy was not performed if one or more standard contraindications occurred (13).
Before the beginning of VIT, patients received a detailed information about it and accepted to perform the therapy for at least 5 years.
In vitro tests
Sera from peripheral blood samples were taken before VIT and were analyzed for venom-specific IgE by Phadebas RAST, according to the supplier's instructions (Pharmacia, Uppsala, Sweden).
VIT
Commercially available aqueous suspensions labeled 100, 1000, 10,000 and 100,000 SQU/mL, corresponding to the allergens from 0.1, 1, 10, 100 μg/mL respectively of raw venom of Vespula spp. adsorbed onto aluminum hydroxide (Alutard SQ, ALK-Abellò), were used during the study. In accordance with the supplier's information, the raw venom was submitted before adsorption to purification on Sephadex with the recovery of the allergen-containing fractions only.
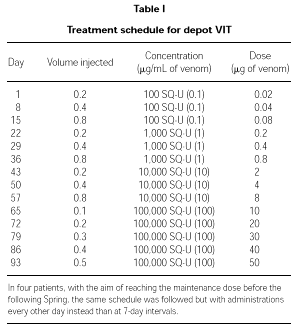
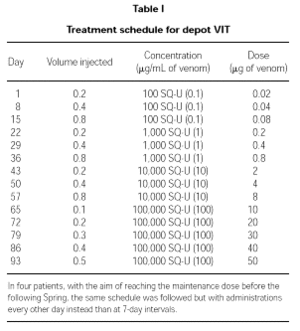
VIT schedule
Induction treatment
Patients were treated with depot VIT reaching a maintenance dose of 50,000 SQU (50 μg) of venom. The schedule followed is detailed in Table I. In four patients who started the therapy too late in Winter to reach the maintenance dose in due time, the induction was performed with the same schedule but with administrations every other day.
Maintenance therapy
Once the maximum dose of 50,000 SQU (50 μg) of venom was reached, maintenance therapy with the same dose of venom was administered after 2, 3 and 4 weeks, and then every 4 weeks during the first year. From the second year on and until the end of the 5-year standard treatment period, administrations were performed every other month.
Premedication
Premedication with one antihistamine tablet (cetirizine or loratadine) to be taken 1 hour before administration was performed only in patients who had shown mild local side effects to the previous administration.
Side effects
Patients were observed for 30-60 minutes after each injection and both local and systemic reactions were recorded. They were also instructed to report delayed reactions to the center immediately, and were interviewed during the following visit to check for any late reaction or discomfort possibly related with therapy. Local side effects were recorded as mild local reactions if erythema and swelling were < 10 cm in diameter, or as large local reactions if erythema and swelling were > 10 cm in diameter. The classification according to the grading I to IV suggested by Mueller (12) was followed for systemic reactions.
Vegetative reactions were not considered to be of allergic origin.
Patients were also instructed to report any sting from the relevant hymenopter and the reactions occasionally occurring from it.
RESULTS
Demographic data
Thirty-six patients (16 females and 20 males) treated on an outpatient regimen and fulfilling the inclusion criteria outlined above were retrospectively identified. Twenty-six of them had had a Grade IV reaction and ten a Grade III reaction to one sting of Vespula spp. before VIT. Three patients did not complete the scheduled administration period, because they stopped the treatment some months after the beginning, for reasons not related to the treatment.
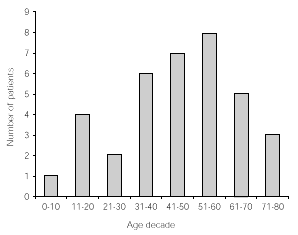
Thirty adults (mean age 53 years, age range 18-73) and three children (6, 11 and 16 years, respectively) followed the treatment for at least 5 years.
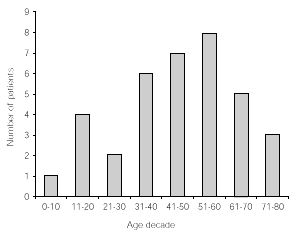
The age distribution of patients included in this study is given in decades in figure 1.
Figure 1.--Age distribution in decades of 33 patients followed up for at least 5 years.
Side effects
No immediate or delayed systemic side effect was detected during the observation period or reported by patients after each administration. Only mild local side effects (erythema and swelling < 10 cm) were reported by 2 patients some hours after the injection during the build-up phase and were controlled by means of local application of ice. One patient had had a Grade III reaction and the other one a Grade IV reaction to the insect sting before VIT.
Efficacy
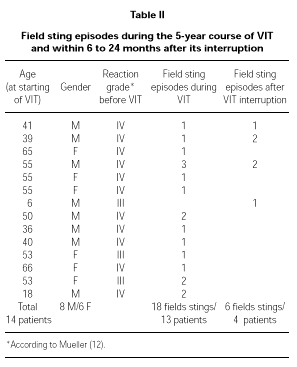
Throughout the 5-year period of VIT, 13 patients (39.4 %) were stung by the relevant insect when already on maintenance therapy. Because some patients have been stung more than once, the total number of stinging episodes were 18. In no case patients experienced systemic symptoms after the sting, but only local mild erythema whereas, before VIT, 11 of them had experienced a Grade IV reaction after a field sting.
Four patients out of 15 who interrupted VIT after the 5-year standard period have been stung between 6 to 24 months after the interruption, reaching a total of six different stinging episodes (one sting in two patients and two stings in two patients). These patients experienced only mild local erythema after each sting, whereas 3 of them had experienced a Grade IV reaction after a field sting before VIT.
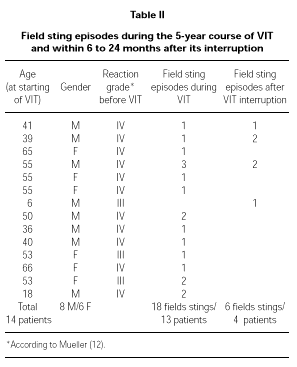
Full data about these patients is shown in Table II.
DISCUSSION
Allergens adsorbed onto aluminum hydroxide are known to be slowly released, with the result of a reduced frequency of early local and systemic side effects in comparison to the corresponding aqueous extract (14), but the available evidence in favor of VIT performed with depot extracts is still poor. Only three studies regarding VIT with purified depot extracts administered according to a weekly schedule are currently available. Wüthrich and coworkers treated 17 patients with depot VIT for Apis, 13 patients with depot VIT for Vespula and 5 with both venoms. Mild systemic reactions occurred only in three patients on Apis VIT (1/139 injections) during the standard 17-week induction phase schedule (9) whereas local large reactions were observed in 1/3 of patients (1/43 injections). Ten out of eleven patients stung again by the relevant insect during the maintenance phase were completely protected and only 1 showed mild (Grade II) symptoms. Mosbech and coworkers reported similar findings. Thirty-two patients allergic to Vespula were divided into 3 groups: 10 patients were treated with aqueous purified extract, 12 with depot purified extract, and 10 with the standard aqueous extract (10). A cluster schedule with administrations twice a week was used for both aqueous extracts, whereas the induction phase with the depot extract took 19 weeks. Few mild systemic reactions occurred in any of the 3 groups. Only minor local reactions were detected when 24 out of 26 patients who completed the first 24 months of treatment were subjected to a sting challenge in the hospital. In another retrospective Italian study, 41 patients sensitized to Vespula spp. were treated for at least three years with a purified depot venom preparation. Only three patients showed mild systemic side effects during the induction phase (top dose 80-100 μg of venom), whereas 7 patients who experienced a field sting during the therapy suffered only from mild local reaction (11). VIT with aqueous extracts is recognized as a life-saving treatment, with a very high degree of success for Apis (75 %) and even higher for Vespula (90 %) (15) but the risk for systemic side effects during aqueous VIT must be anyway considered carefully. The risk is in general higher for Apis than Vespula, but many other variables, like as the age of the patient, the grade of the reaction to natural stings and the administration schedule play a relevant role. Systemic side effects for Apis have been reported in 5-40 % of patients (15), but for some groups of patients and with special administration schedules figures up to 100 % have been also reported (16, 17). Much lower values, between 24 and 45 % for local large side effects and between 6 to 9 % for objective general symptoms, have been reported for Vespula (18-20).
The balance between efficacy and side effects is a key point for VIT, and this aspect has been periodically reviewed (21-24). To increase both safety and compliance, the use of antihistaminics before administrations has been suggested (25, 26).
In this study, we used antihistamine premedication only in 2 patients who had shown local side effects to the previous administration during the build-up phase, mainly to increase compliance.
Local and systemic side effects are mainly related to the induction phase and because of the obvious and marked differences in schedules for aqueous or depot VIT, it is clearly difficult to compare their relative safety because the incidence of side effects depends on the schedule followed (16, 17). In the common practice, aqueous extracts are normally administered according to cluster or rush schedules, whereas the standard schedule for depot extracts involves weekly administration during the induction phase. In both cases maintenance therapy is administered monthly or bimonthly (27).
We selected for our study only patients with very important systemic side effects (Grade III-IV, according to Mueller) after Vespula spp. sting and with a relatively high mean age (49.2 years). According to the literature, these patients are more prone to have important local and systemic side effects both during VIT and after a new sting (20, 28, 29).
We used a maintenance dose of only 50 μg of venom, that is the lowest value in the range recommended for VIT (30, 31). This dose corresponds anyway to several Vespula spp. stings (32), whereas the lowest dose recommended for Apis (100 μg) corresponds to the amount delivered by one or two stings (33, 34). We decided not to follow the variation in IgE and total IgG or subclasses specific to Vespula venom because of the recognized lack of correlation among these parameters and the clinical outcome of VIT (15). During VIT 13 patients experienced a field sting (18 field stings in total) and 4 patients had at least one field sting (6 field stings in total) 6 to 24 months after the interruption of the 5-year treatment. In all of the 24 episodes of field sting patients showed an excellent protection, leading in only a few cases to local mild reaction (erythema). It is important to underline that most of the independent field sting episodes happened in patients who had had a Grade IV reaction before VIT (20 out of 24 total episodes). The same happened for the independent field stings after the interruption of the 5-year course of VIT (4 out of 6).
An excellent tolerance was also shown in four patients following an accelerated schedule (administrations during the induction phase were performed every other day) so as to reach the maintenance dose before the following Spring.
The switch from aqueous to depot preparations has already successfully taken place for inhalant allergens. Our data shows and confirm, in agreement with the available literature, the excellent safety and efficacy also of the purified depot VIT for Vespula spp. in patients with the highest risk profile for both age and systemic reaction grade before VIT.
Our results cannot be of course considered as conclusive, but they increase the body of evidence in favor of the use of purified depot extracts of Vespula spp. as an alternative to the corresponding aqueous extracts, for an improved safety, efficacy and compliance of this life-saving form of therapy.