Inflammation and coagulation are closely linked events. Thrombin is the key enzyme in coagulation system and also has roles in inflammation.
ObjectiveThe aim of our study was to evaluate thrombin generation in children with mild asthma.
MethodsForty-two children with mild asthma and 49 healthy children were included in the study. All patients performed spirometry. Thrombin generation tests (TGT) were performed with a calibrated automated thrombogram (CAT) in children without asthma exacerbation during the last six months. During CAT assay thrombogram curves were obtained. The area under the curve showed endogenous thrombin potentials and indicated the total amount of endogenous thrombin generated; the peak height showed the highest thrombin value, thrombin lag time and time to thrombin peak were measured.
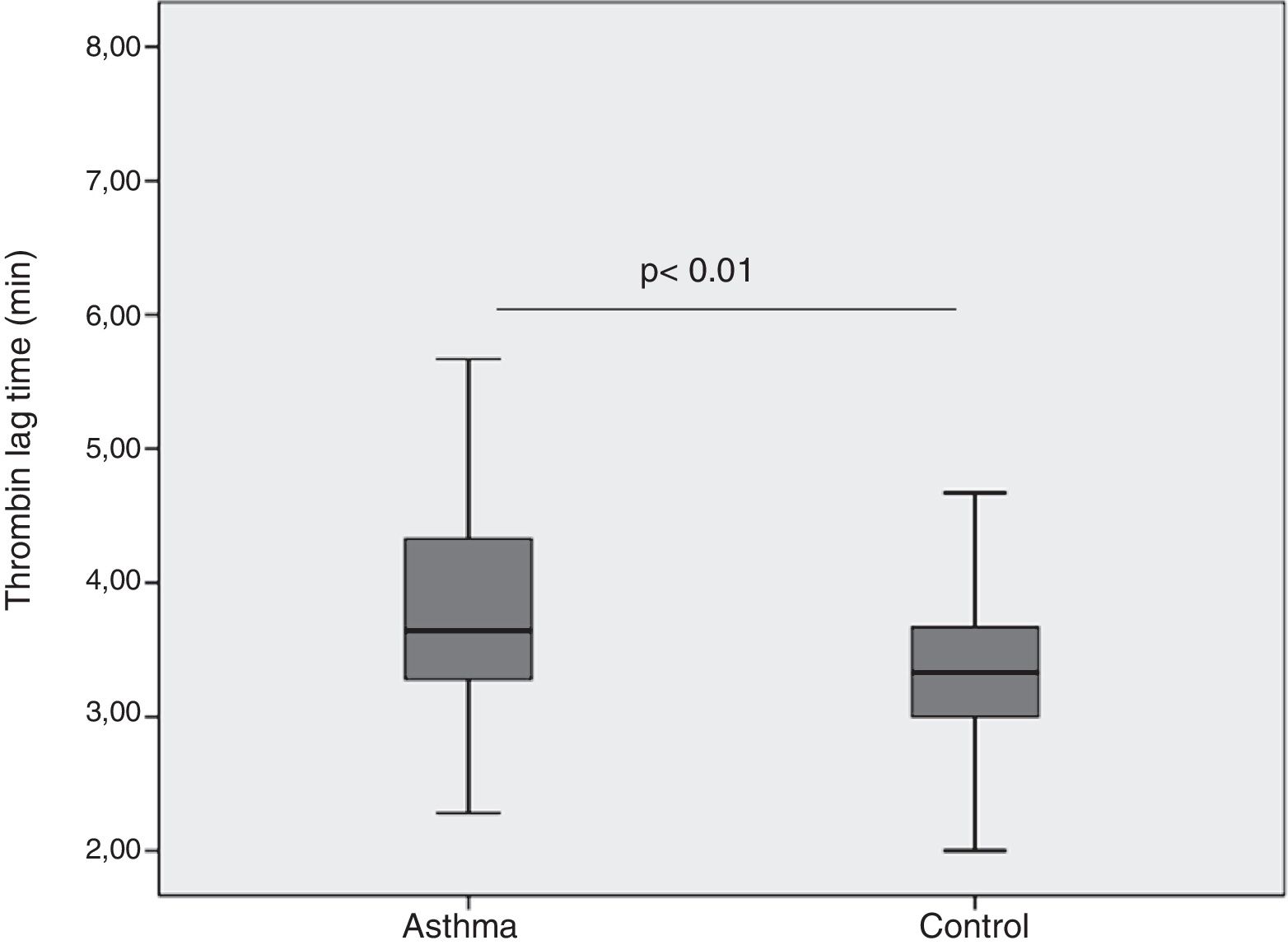
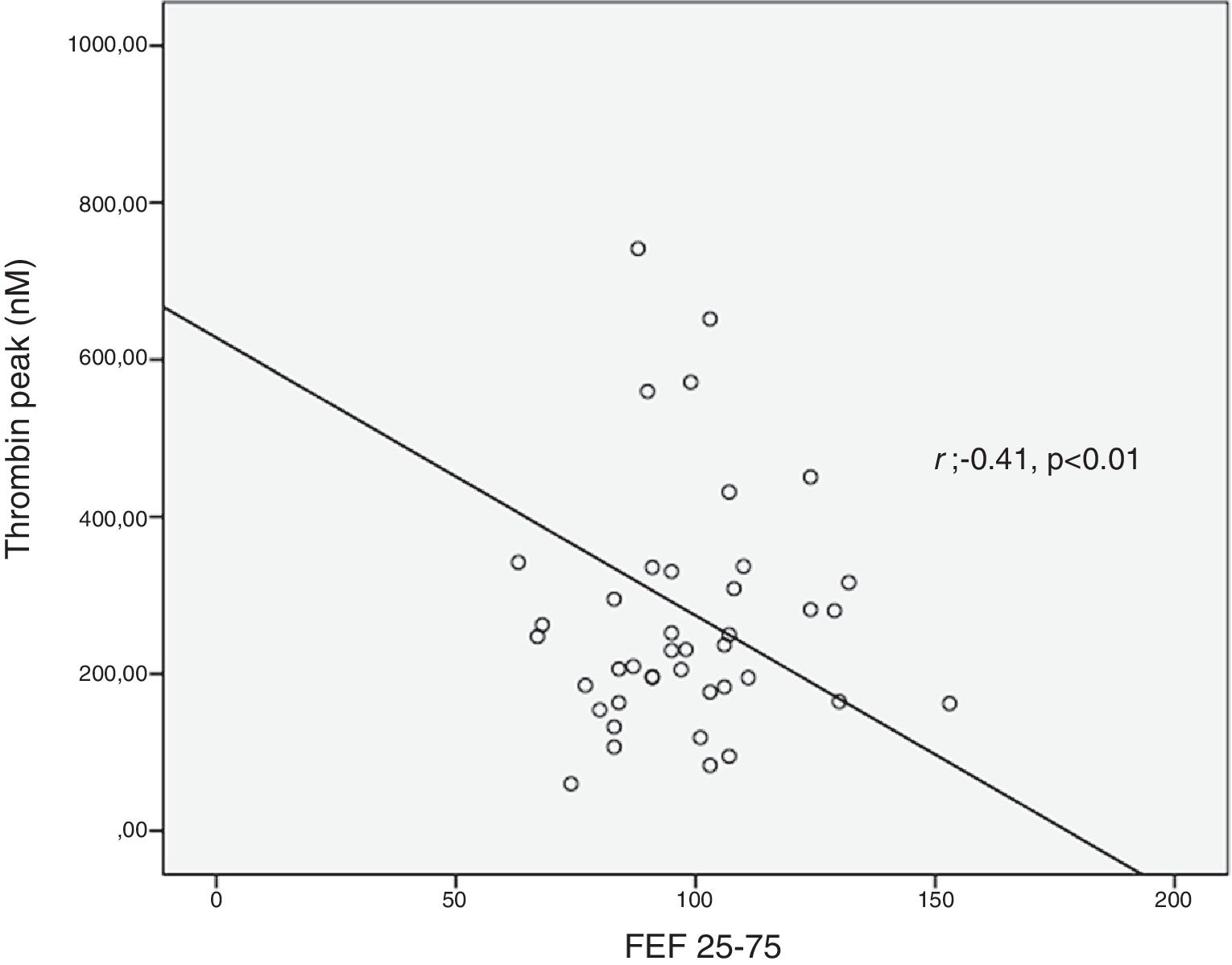
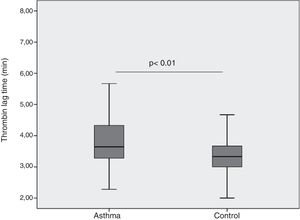
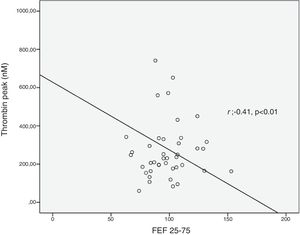
ResultsThrombin lag time was significantly longer in children with asthma (3.98±1.2min) compared to those in the control group (3.29±0.6min) (p<0.01). Children with asthma also had longer thrombin tail time compared to the control group (19.5±8.9min vs. 16.7±2.9min, p=0.02). Thrombin peak was inversely correlated with FEF 25–75 (r=−0.41, p<0.01). Thrombin lag time was inversely correlated with FEF 25–75 (r=−0.39, p<0.01).
ConclusionInflammation in mild asthma seems to disturb coagulation but this disturbance may not be so strong as to increase thrombin levels and may only affect the initiation phase of thrombin generation.
Inflammation and coagulation are closely linked events. Inflammatory cytokines are major mediators involved in the activation of coagulation, ultimately causing a tendency towards coagulation in patients who have inflammatory disorders.1–3 Disturbed hemostatic balance and coagulation activation have been demonstrated in airways of patients with asthma.4–6 Thrombin is the key enzyme in coagulation system. In addition to its well-known effect, transforming fibrinogen to fibrin, it has a role in stimulating the secretion of inflammatory mediators and cytokines.7 Thrombin induces inflammation by activation of protease activated receptors and protease activation has been shown to induce allergic inflammation.7 Elevated thrombin concentrations in airways of asthma patients has been shown in recent studies.4,8,9 An association between severe asthma and increased risk of pulmonary thromboembolism is also revealed.8–12 In this study, we aimed to evaluate thrombin generation in children with mild asthma. We also intended to demonstrate associations between thrombin levels and control of asthma in our patients.
MethodsPatientsForty-two children (male/female: 24/18) with mild asthma and 49 (male/female: 31/18) healthy children who were under the age of 16 were included in the study. Fifteen patients (36%) had intermittent and 27 patients (64%) had mild persistent asthma. All children with asthma had no asthma exacerbation during the previous six months. Asthma was diagnosed according to the Global Strategy for Asthma Management and Prevention (GINA) classification. It is based on a history of intermittent wheezing and demonstration of reversible airway obstruction as defined by at least a 12% improvement in forced expiratory volume in 1s (FEV1) following bronchodilator administration.13 Severity and control of asthma were assessed according to GINA guidelines.13 Intermittent asthma was defined as children treated only with inhaled short acting β2-agonists on demand and not receiving inhaled corticosteroids (ICS) or another asthma control treatment. Mild persistent asthma was defined as children treated with a low daily dose of inhaled corticosteroids or daily montelukast.
Children with asthma were classified according to GINA as well-controlled, partly controlled or uncontrolled.13 Asthma control was also assessed by asthma control test (ACT). An ACT score 20–25 indicated well-controlled asthma, 16–19 indicated not well controlled and 5–15 indicated poorly controlled asthma.
Pulmonary function tests were performed with spirometry (Flowhandy Spirometer ZAN 100, ZAN Messgeräte GmbH, Germany) just before venopuncture. Forced vital capacity (FVC), FEV1, FEV1/FVC and forced expiratory flow (FEF25–75) were measured. An adequate test required a minimum of three acceptable FVC manoeuvres. While performing spirometry, a minimum of three flow-volume loop results has been obtained. Acceptable repeatability is achieved when the difference between the largest and the next largest FVC is ≤0.150L and the difference between the largest and next largest FEV1 is ≤0.150L. Skin prick test results were obtained from the patients’ medical records.
Thrombin generation testsVenous blood was drawn into standard tubes containing sodium citrate for thrombin generation tests (TGT). Following a centrifugation at 5000×g for 25minutes (min) at room temperature, platelet poor plasma (PPP) was collected from the upper half volume of plasma supernatant and rapidly frozen at −80°C. Thrombin generation tests were performed with a calibrated automated thrombogram (CAT®, Thrombinoscope BV, Maastricht, the Netherlands) device that uses a slow-acting fluorogenic substrate. In TGTs, thrombin generation occurs in the presence of both phospholipid and tissue factor. A sample of 80μL PPP was mixed with 20μL of a mixture containing tissue factor (Dade-Behring) at a final concentration of 1pM and phospholipid vesicles.
Each sample was transferred to three different microtitrated plates (Immulon 2HB, Thermo Electron Corporation, Milford, MA, USA) that involved 80μL of plasma and 20μL of thrombin calibrator. After the incubation of the mixture at 37°C for 15min, a sample of 20μL was added to 20μL of Fluo-Buffer® solution, and the reaction was monitored with a fluorometer.
Using the Trombinoscope® programme, thrombogram curves were obtained. Endogenous thrombin potentials (ETP), peak heights, thrombin lag time and time to thrombin peak were measured. The area under the curve, which indicates the total amount of endogenous thrombin generated (ETP) and the peak height during the recording time (thrombin peak) which indicates the highest thrombin value were recorded. “Lag time” was the time from the start of analysis to time that thrombin started to generate and “time to thrombin peak” was the time from the start of thrombin generation until the maximum thrombin value. During CAT assay thrombin tail time which described the time that the curve reaches the end and showed thrombin cessation, were also measured and recorded.
This study was approved by Baskent University Ethical Committee (KA16/30) and written informed consent was obtained from all subjects or parents prior to the study.
Statistical analysisData analyses were performed using the SPSS Statistics for Windows v20.0 (SPSS, Inc., Chicago, IL, USA). Shapiro–Wilk's test was used to assess the normality of distributions of the variables, and Levene's test was used to assess the homogeneity of variances among groups. Normally distributed and homogeneous variances groups were compared, two groups by Student's t test and three or more groups by Analysis of Variance. Categorical data was analyzed with Fischer's Exact Test and Chi-square test. According to the test results, parametric test assumptions were not available for some variables, so the comparisons of two independent groups were performed by Mann–Whitney U test, comparisons of three independent groups were performed by Kruskal Wallis test. The Pearson correlation coefficient was calculated to evaluate the relationships between variables. Descriptive statistics were reported as frequencies and percentages for categorical variables and as mean±standard deviation (SD) for continuous variables. Data that have a skewed distribution are presented as medians and interquartile ranges. A p-value<0.05 was considered as statistically significant.
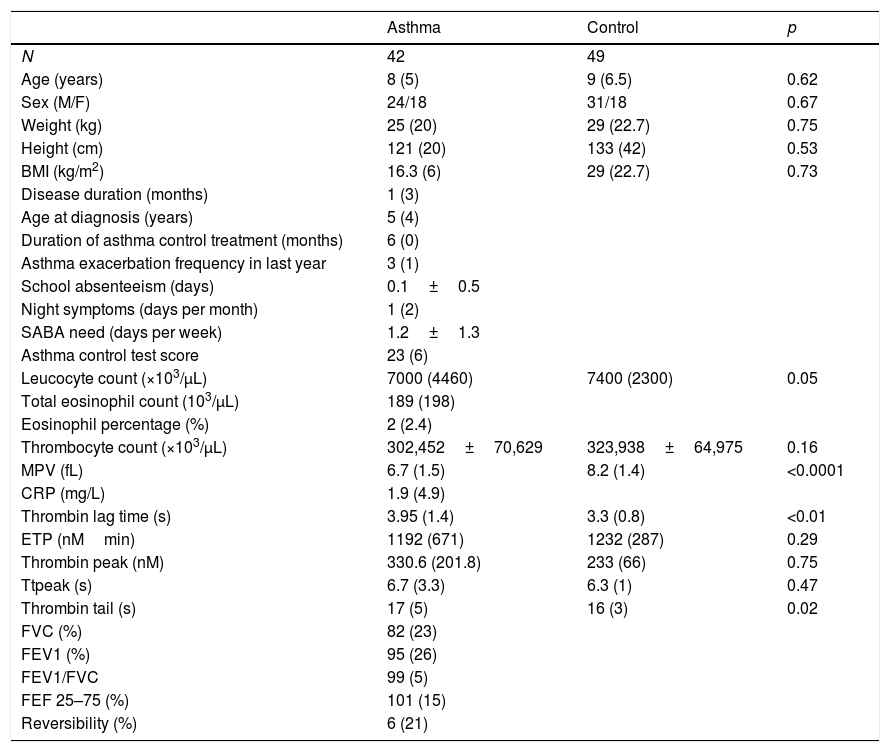
ResultsIn patients with asthma, median age at diagnosis was five years and median duration of the asthma control treatment was six months. The demographic and clinical characteristics of participants in the study are summarized in Table 1.
Characteristics of participants in the study.
| Asthma | Control | p | |
|---|---|---|---|
| N | 42 | 49 | |
| Age (years) | 8 (5) | 9 (6.5) | 0.62 |
| Sex (M/F) | 24/18 | 31/18 | 0.67 |
| Weight (kg) | 25 (20) | 29 (22.7) | 0.75 |
| Height (cm) | 121 (20) | 133 (42) | 0.53 |
| BMI (kg/m2) | 16.3 (6) | 29 (22.7) | 0.73 |
| Disease duration (months) | 1 (3) | ||
| Age at diagnosis (years) | 5 (4) | ||
| Duration of asthma control treatment (months) | 6 (0) | ||
| Asthma exacerbation frequency in last year | 3 (1) | ||
| School absenteeism (days) | 0.1±0.5 | ||
| Night symptoms (days per month) | 1 (2) | ||
| SABA need (days per week) | 1.2±1.3 | ||
| Asthma control test score | 23 (6) | ||
| Leucocyte count (×103/μL) | 7000 (4460) | 7400 (2300) | 0.05 |
| Total eosinophil count (103/μL) | 189 (198) | ||
| Eosinophil percentage (%) | 2 (2.4) | ||
| Thrombocyte count (×103/μL) | 302,452±70,629 | 323,938±64,975 | 0.16 |
| MPV (fL) | 6.7 (1.5) | 8.2 (1.4) | <0.0001 |
| CRP (mg/L) | 1.9 (4.9) | ||
| Thrombin lag time (s) | 3.95 (1.4) | 3.3 (0.8) | <0.01 |
| ETP (nMmin) | 1192 (671) | 1232 (287) | 0.29 |
| Thrombin peak (nM) | 330.6 (201.8) | 233 (66) | 0.75 |
| Ttpeak (s) | 6.7 (3.3) | 6.3 (1) | 0.47 |
| Thrombin tail (s) | 17 (5) | 16 (3) | 0.02 |
| FVC (%) | 82 (23) | ||
| FEV1 (%) | 95 (26) | ||
| FEV1/FVC | 99 (5) | ||
| FEF 25–75 (%) | 101 (15) | ||
| Reversibility (%) | 6 (21) |
BMI, body mass index; CRP, C-reactive protein; ETP, endogenous thrombin potential; F, female; FEF, forced expiratory flow; FEV1, forced expiratory volume in 1s, FVC, forced vital capacity; M, male; min, minute; MPV, mean platelet value; SABA, short acting beta-2-agonist; Ttpeak, time to thrombin peak.
For non-normally distributed variables, values are shown as median (interquartile range) and for normally distributed variables, values are shown as mean±SD.
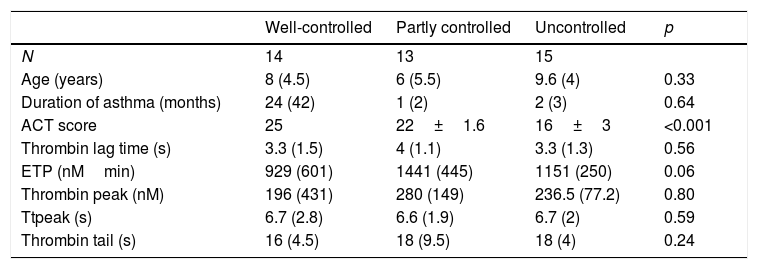
Asthma of 14 (33.3%) patients was well-controlled, however, asthma of 15 (35.7%) patients was uncontrolled (Table 2). Analysis spirometry was performed just before taking blood for TGT, and the results of spirometric measures are shown in Table 1. Platelet counts and mean platelet volumes (MPV) of the patient (302,452±70,629×103/μL; 7.1±0.9fL) and control groups (323,938±64,975×103/μL; 8.4±1.2fL) showed no difference in terms of platelet counts, but the difference was significant for MPV (p<0.001).
Thrombin generation test parameters according to asthma control.
| Well-controlled | Partly controlled | Uncontrolled | p | |
|---|---|---|---|---|
| N | 14 | 13 | 15 | |
| Age (years) | 8 (4.5) | 6 (5.5) | 9.6 (4) | 0.33 |
| Duration of asthma (months) | 24 (42) | 1 (2) | 2 (3) | 0.64 |
| ACT score | 25 | 22±1.6 | 16±3 | <0.001 |
| Thrombin lag time (s) | 3.3 (1.5) | 4 (1.1) | 3.3 (1.3) | 0.56 |
| ETP (nMmin) | 929 (601) | 1441 (445) | 1151 (250) | 0.06 |
| Thrombin peak (nM) | 196 (431) | 280 (149) | 236.5 (77.2) | 0.80 |
| Ttpeak (s) | 6.7 (2.8) | 6.6 (1.9) | 6.7 (2) | 0.59 |
| Thrombin tail (s) | 16 (4.5) | 18 (9.5) | 18 (4) | 0.24 |
ACT, asthma control test; ETP, endogenous thrombin potential; Ttpeak, time to thrombin peak.
For non-normally distributed variables, values are shown as median (interquartile range) and for normally distributed variables, values are shown as mean±SD.
When we compared patient and control groups, no difference in thrombin peak, time to thrombin peak and ETP have been obtained (Table 1). Yet, thrombin lag time was significantly longer in children with asthma (3.98±1.2min) compared to those in control group (3.29±0.6min) (p<0.01) (Fig. 1). Children with asthma also had longer thrombin tail time compared to control group (19.5±8.9min vs. 16.7±2.9min, p=0.02).
Thrombin generation parameters did not show differences according to asthma severity (mild intermittent vs. mild persistent), asthma control levels, asthma control scores, having atopy and using ICS or montelukast (Table 2). C-reactive protein (CRP) was measured in 26 patients and did not show correlation with thrombin generation parameters. Thrombin peak was inversely correlated with FEF 25–75 (%) (r=−0.41, p<0.01) (Fig. 2). Thrombin lag time was inversely correlated with FEF 25–75 (%) (r=−0.39, p<0.01). Thrombin peak was inversely correlated with FEV1/FVC (r=−0.34, p<0.05).
DiscussionThrombin is a multifunctional protein involves in coagulation, anticoagulation, platelet activation, endothelial activation, production of growth factors and proliferation of both smooth muscle cells and fibroblasts.14 It is also a mediator of inflammation in diseases – including asthma, rheumatoid arthritis, and cancer.15 Therefore, in patients with asthma disturbance in thrombin, kinetics would be expected.
Patients with asthma have a prothrombotic state that increases with asthma severity and have an altered thrombin generation profile compared with controls.16,17 Higher ETP and peak thrombin levels were found in patients with asthma, and peak thrombin levels have been demonstrated to increase with asthma severity.16,17 As our patients had mild asthma, thrombin levels may not have been affected. On the other hand, airway inflammation in asthma is controlled with asthma treatments. Recently, the prothrombotic state in asthma has been shown to be related to increased levels of inflammatory cytokines, IL-6 and TNFα, in peripheral blood.18 As far as we know, this is the first study investigating thrombin generation parameters in children with asthma. In our study, thrombin lag time and thrombin tail time were longer in children with mild asthma whereas thrombin generation parameters including thrombin peak, ETP and time to thrombin peak in children with mild asthma were similar with healthy children. These results are compatible with similar/lower thrombin generation tests which is on the contrary of our hypothesis of increased thrombin generation in asthma. However, since lag-time is the initiation phase of the thrombin generation and tail time is the end phase which shows the final inhibition of thrombin, showing longer lag and tail times may indicate that coagulation time is affected even in children with mild asthma.
Our patients with mild persistent asthma were using either inhaled steroid or montelukast which may have prevented a rise in thrombin levels by controlling inflammation. Similar to our study, Sneeboer et al. demonstrated that there was a longer lag time in adult asthmatic patients compared with healthy control subjects and ETP levels did not show difference between patients with mild asthma and controls.16 However, thrombin peak time was inversely correlated with both FEF 25–75 and FEV1/FVC values. Since our patients had no asthma exacerbation during the previous six months, similar/lower values may depend on the suppression of inflammation by drugs. Inverse correlations between thrombin levels and both FEF 25–75 and FEV1/FVC values are still compatible with our hypothesis. These results reveal increased thrombin generation can be associated with decreased spirometric values including FEF 25–75 and FEV1/FVC. This situation can be explained by bronchoconstriction in lower airways. Rising thrombin levels which cause increase in inflammation and excessive fibrin deposition may contribute to airway narrowing. Fibrin is the end product of coagulation and is generated after cleavage of fibrinogen by thrombin. Elevated fibrin concentrations in the airways can produce lung function disorder characteristics for asthma.6,19 Thrombin lag time was also inversely correlated with FEF 25–75. We think that the factors increasing inflammation may cause an increase in lag time.
In a recent study, Kremers et al. found that the ETP mainly depends on the total amount of converted prothrombin and the thrombin decay capacity.20 The peak is determined by the maximum rate of prothrombin conversion and the thrombin decay capacity.20 We think that coagulation/anticoagulation balance is disturbed in mild asthma, but this disturbance may not be so strong as to increase thrombin levels and may only cause an increase in lag and tail times.
Having a small sample size, the absence of other measures of plasma thrombin formation, antithrombin and inflammatory cytokines that can affect prothrombotic state are limitations of our study. Searching other measures of thrombin formation and determinants of altered lag phase including antithrombin and inflammatory markers would have given more reliable results. Future prospective studies with these parameters would help to shed light on unexplained parts about this subject.
In conclusion, thrombin levels are not affected in children with mild asthma in our study. However, there is an inverse correlation between FEF 25–75, FEV1/FVC values and thrombin levels in our patients indicating airway obstruction with the increased thrombin levels. We believe that the present study could be of interest given growing evidence for the involvement of a prothrombotic state in various aspects of asthma. Since this study has been carried out in mild asthmatic patients who had no asthma exacerbation during the previous six months, further study in children with moderate/severe asthma including asthma exacerbation period could give more reliable results.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflict of interest to declare.