The present study was designed to assess the function of tissue plasminogen activator (t-PA) expression in allergic rhinitis.
MethodsAge-matched t-PA gene knock out (t-PA-/-) and wild type (WT) mice were sensitised four times, and then challenged for six weeks with ovalbumin. The controls were treated with saline instead of ovalbumin. The structural change in the nasal mucosa was investigated with haematoxylin and eosin stain and van Gieson staining. u-PA (urokinase-type plasminogen activator) and PAI-1 (plasminogen activator inhibitor) gene expression were measured by real time PCR. Matrix metalloproteinase-9 (MMP-9) expression was tested with Western blotting and with real time PCR.
ResultsAfter ovalbumin challenge for six weeks, compared with the WT group, t-PA depletion increased collagen deposition and gland hyperplasia. u-PA and PAI-1 gene expression increased both in t-PA-/- and in WT mice after ovalbumin treatment. MMP-9 expression decreased greatly after ovalbumin challenge in t-PA-/- mice.
Conclusiont-PA affects the nasal mucosa matrix reconstruction process in allergic rhinitis, with which MMP-9 is involved.
Allergic rhinitis (AR) is one of the more frequently encountered diseases in the otolaryngology department. Its prevalence is between 10% to 40% in the global population.1 Previous studies on the remodelling of the nasal airway in AR have proved that the allergic inflammatory response results in the remodelling of the epithelium and the basement membrane; although some contradictory results have also been reported.2,3 Sanai et al. reported that there was extensive immunoreactivity for collagen type I and II deposited on the reticular basement membrane in allergic subjects.4
The fibrinolytic system includes serine proteases and their inhibitors. Tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) are two capital serine proteases, and PAI-1 is the predominant physiological inhibitor of t-PA and u-PA. Studies have proven that fibrinolytic components, including t-PA, are not only associated with proteolytic cascade in thrombolysis; but also that they play an essential role in other fibrinolysis biological processes, including tissue remodelling, tumour metastasis, and inflammation.5–7 PAs (plasminogen activators) activate plasminogen into plasmin, which degrades matrix components or amplifies the proteolytic potential by activating pro-matrix metalloproteinases (pro-MMPs) into MMPs.8 In allergic rhinitis patients, Sejima et al. reported that t-PA mRNA increased in the allergic nasal mucosa, and they suspected that t-PA might help modify the watery nasal discharge in AR.9 Given a value of t-PA for the equilibrium of extracellular matrix production and degradation, we conceive that the dysregulation of t-PA might affect the pathogenesis of nasal mucosa remodelling in AR.
To further evaluate the relationship between t-PA and the local nasal airway, we duplicated the ovalbumin (OVA) sensitised AR model in t-PA gene knock out (t-PA-/-) and wild type (WT/t-PA+/+) mice. Subsequently we investigated the structure change of nasal mucosa and the expression of urokinase-type plasminogen activator (u-PA), plasminogen activator inhibitor (PAI-1) and matrix metalloproteinase (MMP-9). The results validate that t-PA plays an important role in the nasal mucosa reconstruction in allergic rhinitis, and MMP-9 might take part in this process.
Material and methodsAnimals and OVA sensitisationC57BL/6J t-PA-/- and WT (t-PA+/+) mice were donated by The Key Laboratory of Molecular Medicine at Fudan University. Mice were housed in a pathogen-free facility (12hours light and 12hours dark cycle). The genotypes of experiment animals were tested by PCR analysis on the DNA obtained from the tail. The animals were treated according to local guidelines for animal care.
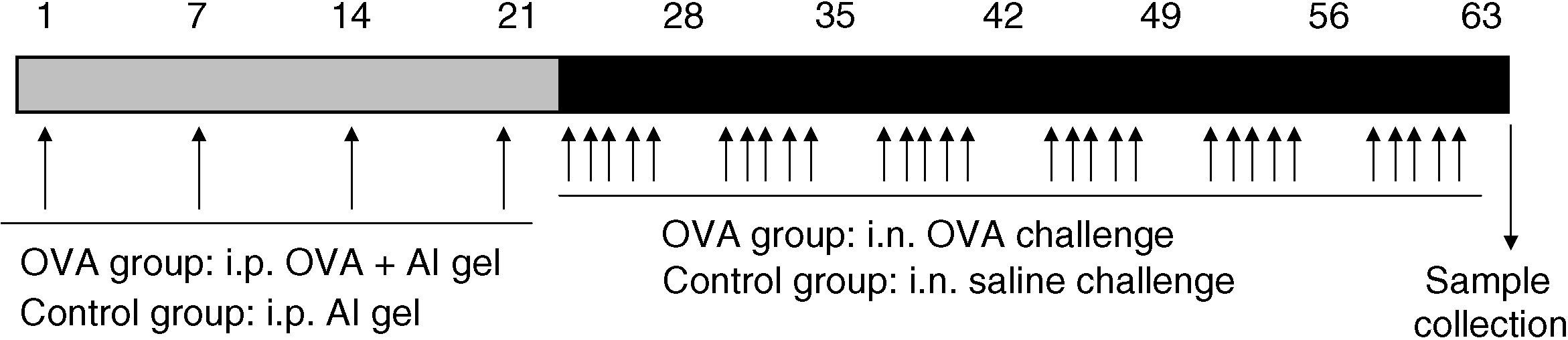
t-PA-/- or WT mice (eight weeks old, male) were randomly divided into four groups, and treated as previously described10: 1) The OVA-treated t-PA-/- group (n=8); 2) The OVA-treated WT group (n=8). Both OVA-treated groups were given OVA sensitisation by intraperitoneal (i.p.) injection and then received OVA by intranasal (i.n.) challenge five times a week for six weeks. 3) The saline-treated t-PA-/- group (n=8); 4) The saline-treated WT group (n=8). Both saline-treated groups were given the same dosage of normal saline instead of OVA. Mice in the OVA group were treated as follows: 10μg/mouse OVA with 1mg/mouse aluminium hydroxide gel were mixed and administered by i.p. on days 1, 7, 14, and 21; and followed by i.n. challenge with 20μl of 25mg/ml OVA per mouse five times a week for six weeks (Figure 1).
Protocol for OVA sensitisation and challenge. Matched mice were i.p. injected OVA and saline aluminium hydroxide gel solution on days 1, 7, 14, 21. This was followed by nasal drops containing OVA and saline five times per week in six weeks. After the last treatment, mice were sacrificed and nasal mucosa samples collected.
After the last i.n challenge, the mice were transcardially perfused with ice-cold PBS (40ml) under deep anaesthesia. Then the mice were decapitated. The nose mucosa was removed and immediately frozen in dry ice (five mice for each genotype in every group). Half of the mixed mucosa was placed in TRIzol (invitrogen), and the other was placed in protein extraction agent. The remaining three mice were decapitated and every intact nose was fixed in 4% paraformaldehyde and then decalcified in 10% EDTA (Ethylene Diamine Tetraacetic Acid) solution.
Histological staining and analysisAfter fixation, the specimens were embedded in paraffin and cut into 5μm thick sections. The sections were stained with haematoxylin and eosin (HE), and van Gieson (VG) staining agents. The sections were digitised and recorded with a Fuji film microscope system.
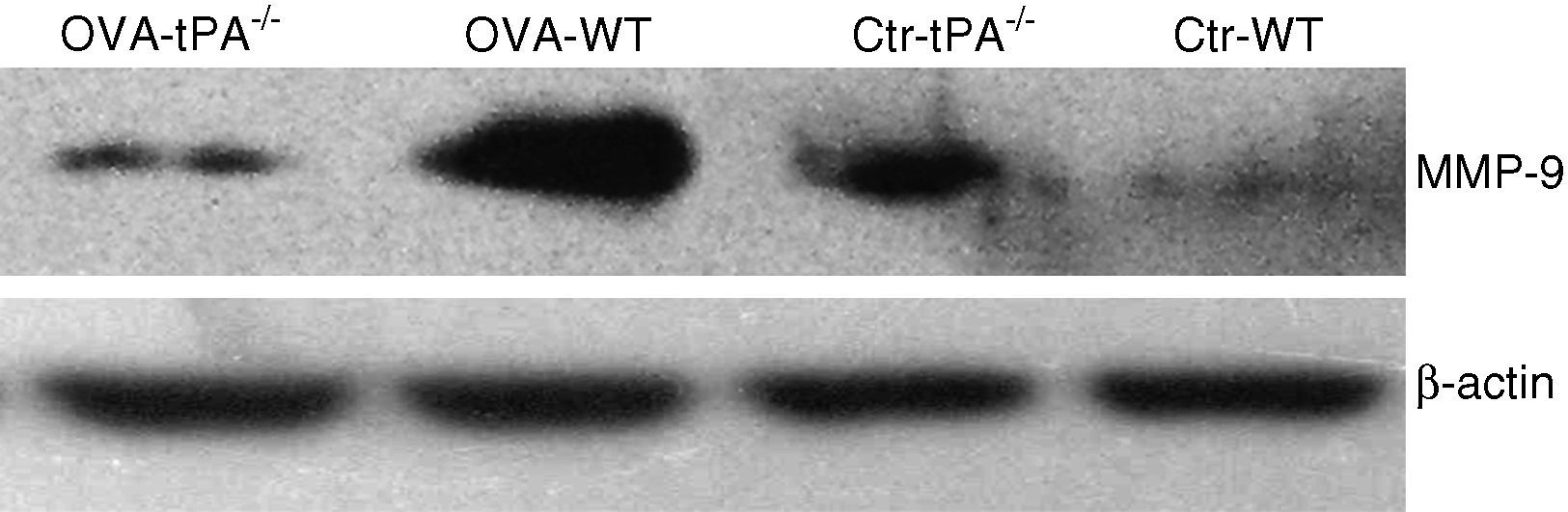
Western blottingTotal tissue protein was extracted by tissue protein extraction solution (Pierce). Protein concentration was quantified by bicinchoninic acid (BCA) protein kit (Pierce corp.). Aliquots of 25μg protein were added to 12% polyacrylamide gel. After electrophoresis, the samples were transferred to PVDF (polyvinylidene difluoride) membrane, and blocked in 5% non-fat milk overnight at 4°C. Then, membranes were incubated with rabbit anti-mouse MMP-9 (1:1000, Chemicon) and β-actin (1:1000, Sigma) antibodies for one hour at room temperature. Membranes were washed and incubated for one hour with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies. The proteins were visualised with Supersignal West Pico Chemiluminescent substrate (Pierce) and developed on X-ray film.
Real-time Quantitative PCRThe nasal mucosa was resuspended in 1ml of TRIzol solution for RNA extraction. First-strand cDNA was transcribed by reverse transcription (RT) kit (Takara) from 2μg of total RNA using random Oligo dT primers. After reverse transcription, total DNA was diluted by ddH2O for quantitative real time-PCR. Quantitative cDNA amplification was performed using an ABI 7300 real-time PCR machine (Applied Biosystems), and PCR conditions as specified by the manufacturer. A real-time PCR kit (Takara) was used in the PCR process. Gene-specific primers were designed by primer3. u-PA primer: 5’-TTA CTG CAG GAA CCC TGA CAA CCA-3’(sense), 5’-TGC TAA GAG AGC AGT CAT GCA CCA-3’(antisense); PAI-1 primer: 5’-CAA TGT GTC ATT TCC GGC TGC TGT-3’(sense), 5’-TTG ACC AGG AGC TGC TGT CTC TTT-3’(antisense); MMP-9 primer: 5’-CCT GTA AAT CTG CTG AAA CC-3’ (sense), 5’-TCT GAC CTG AAC CAT AAC G-3’ (antisense); GAPDH primer: 5’-TCA ACG GCA CAG TCA AGG-3’ (sense), 5’-ACT CCA CGA CAT ACT CAG C-3’ (antisense). After real time PCR amplification, PCR products of u-PA and PAI-1 primers were resolved in 2.5% agarose gels, stained with ethidium bromide, and photographed under UV light.
Statistical analysisValues were expressed as mean±SEM. Statistical analysis was performed using student's t-test and ANOVA, followed by post hoc analysis with Bonferroni's test. Statistical significance was assigned at p<0.05. All analyses were performed using SPSS 11.5 software.
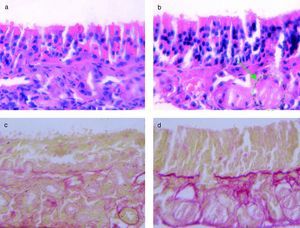
ResultsHistological analysis of the nasal mucosaWe studied the structure changes in t-PA-/- mice and WT mice by HE and VG staining, as described above. After six weeks of intranasal challenge with OVA, the nasal mucosa was dissected and stained (Figure 2).
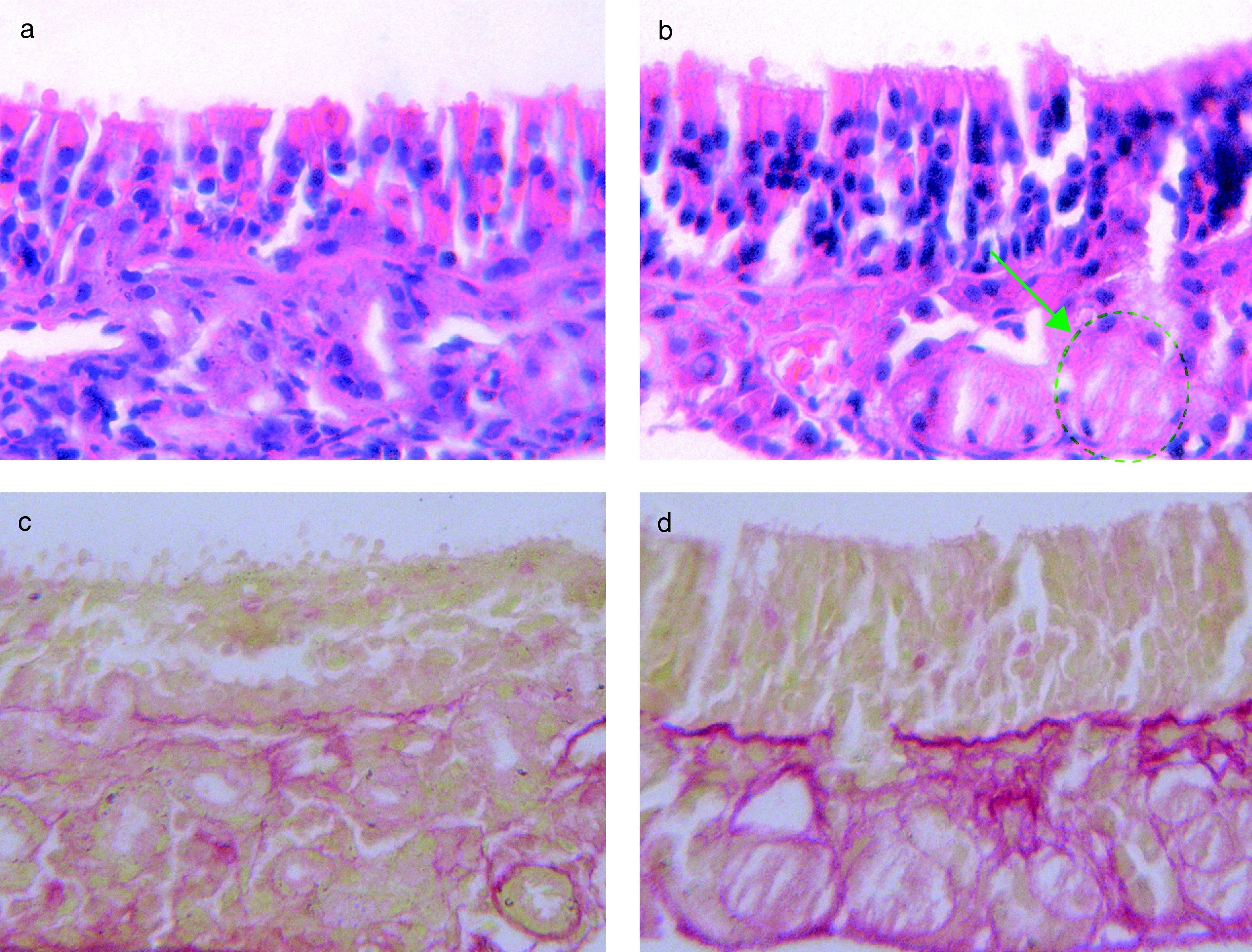
(a) HE stain of OVA-challenged WT mice. (b) The HE stain of OVA challenged t-PA-/- mice, with gland structure disorders (green circle). (c) VG stain of OVA-challenged WT mice, with little collagen fibre deposition (red stained area). (d) VG stain of OVA-challenged t-PA-/- mice, with a lot of collagen fibre deposition (red stained area).
In the saline treated t-PA-/- and WT mice, the mucosa structure had no significant difference (data not shown). In the OVA-challenged, the mucosa from t-PA-/- mice had severe gland structure disorders in HE stain sections (Figure 2b). Additionally, the VG staining showed that there were significant collagen deposits on the nasal mucosal membrane of the t-PA-/- mice (Figure 2d).
u-PA and PAI-1 mRNA in nasal mucosa
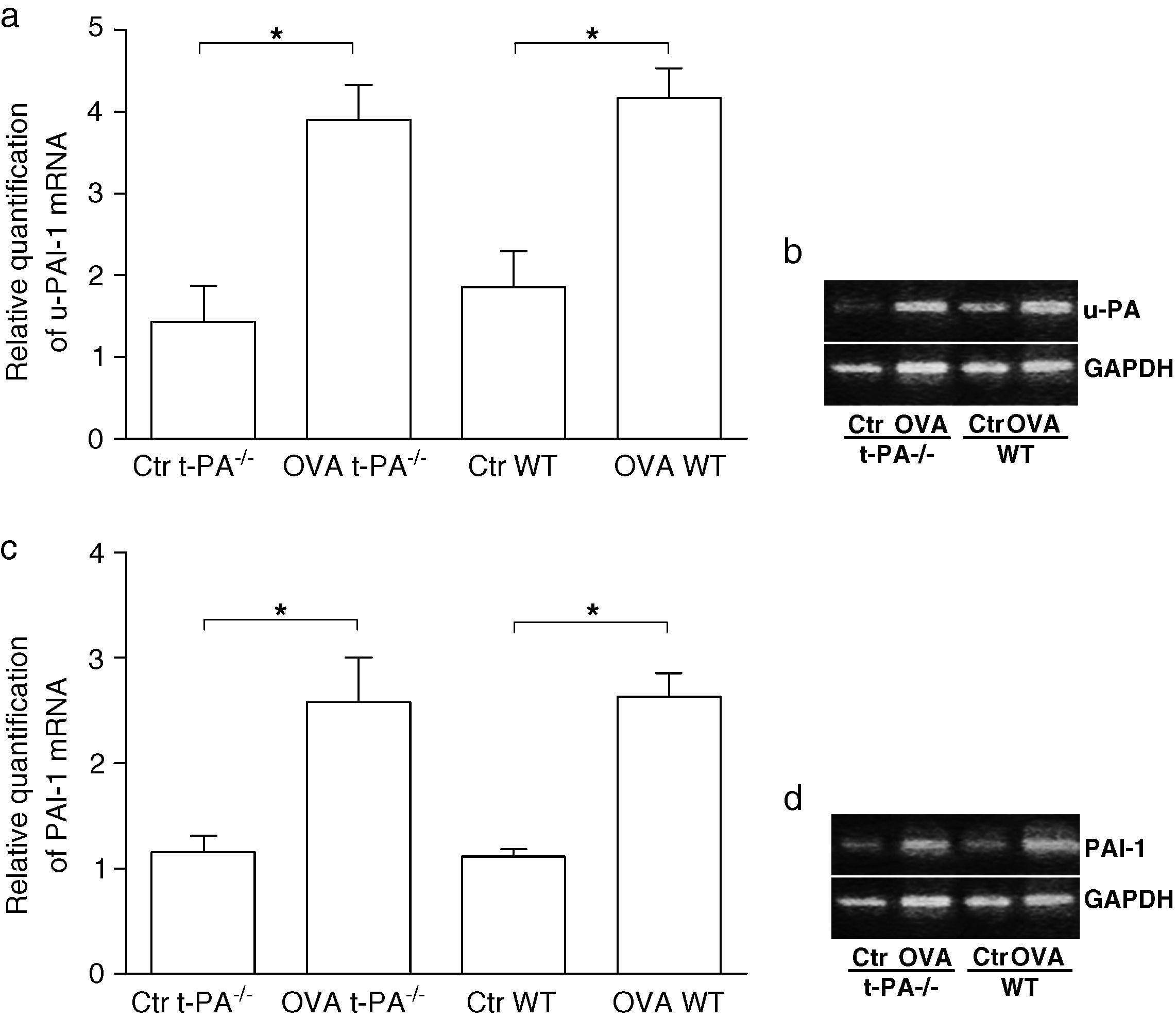
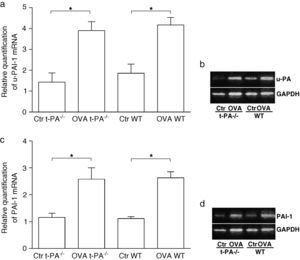
To determine whether the expression of fibrinolytic components changed in nasal mucosa of t-PA-/- and WT mice after being treated with OVA, the mRNA of u-PA and PAI-1 were tested using real time PCR. As shown in Figure 3, after OVA treatment, u-PA and PAI-1 mRNA significantly increased both in the t-PA-/- and WT mice.
Quantitative real-time RT-PCR detection of u-PA and PAI-1 mRNA in saline- (Control, Ctr) and OVA-treated t-PA-/- and WT mice nasal mucosa. (a) The u-PA mRNA quantitative, (c) the PAI-1 mRNA quantitative. In addition, the electrophoretic patterns of PCR products are shown in b and d. (n=5 each, *p<0.05).
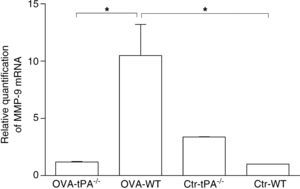
Previous studies have shown that t-PA works mainly in fibrin homeostasis.11 The collagen deposit in t-PA-/- mice might be mediated by other extracellular collagen catabolic enzymes. MMP-9 is an extensively expressed collagen catabolic enzyme found in the extracellular matrix. We tested the MMP-9 gene expression in t-PA-/- and WT mice using real time PCR (Figure 4).
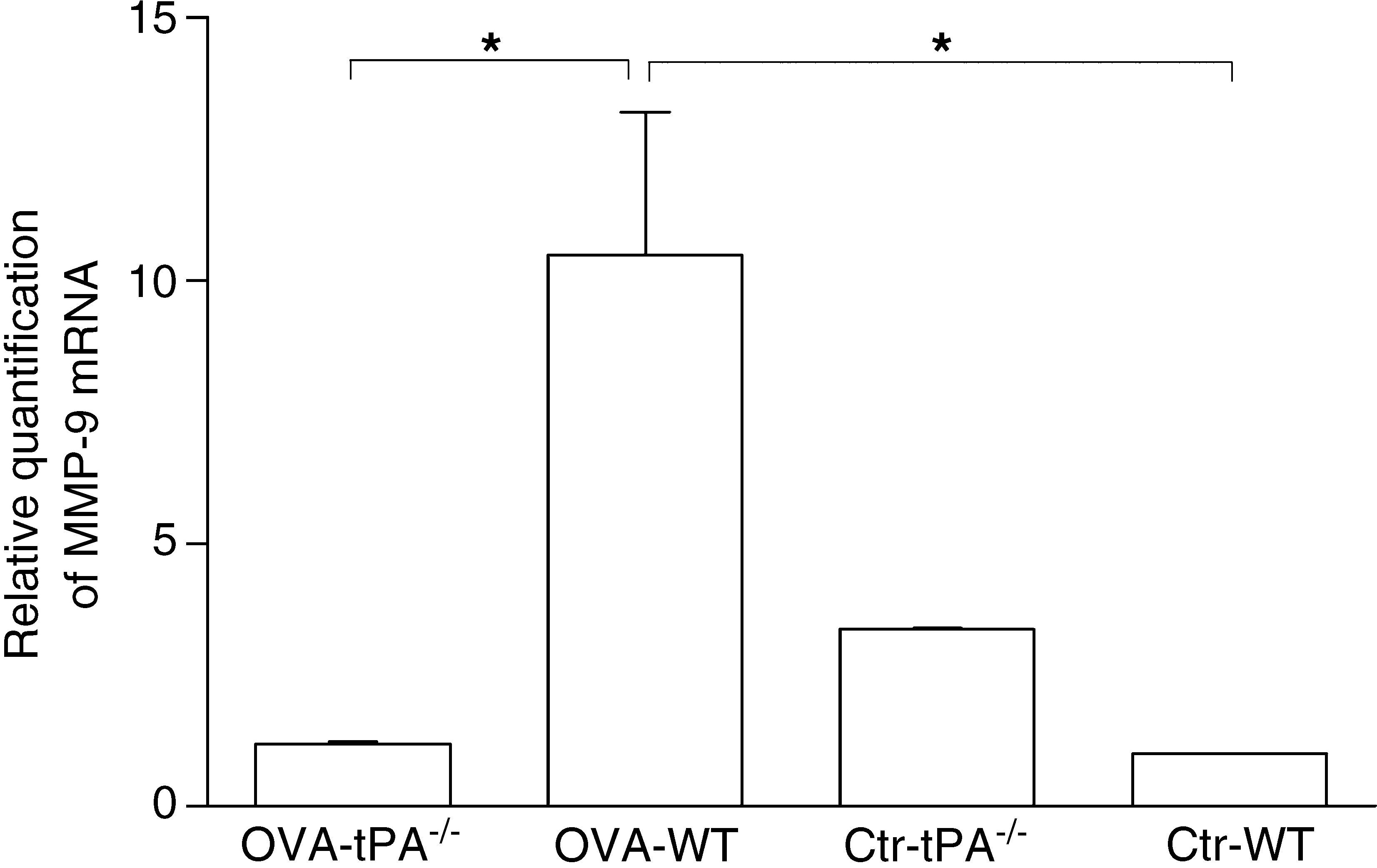
Real-time PCR detection of MMP-9 mRNA in OVA-challenged and saline-treated (Control, Ctr) t-PA-/- and WT mice. The OVA-challenged t-PA-/- mice have little MMP-9 mRNA expression compared with OVA-challenged WT and saline control t-PA-/- mice. Ctr-t-PA-/-: saline-treated t-PA-/- mice, OVA-t-PA-/-: OVA-treated t-PA-/- mice, Ctr-WT: saline-treated WT mice, OVA-WT: OVA-treated WT mice. (n=5 each, * p<0.05).
After six weeks of OVA challenge, the WT mice had a significant increase of MMP-9 gene expression compared to OVA-challenged t-PA-/- mice. However, in the saline treated groups, MMP-9 gene expression was lower in WT mice than t-PA -/- mice.
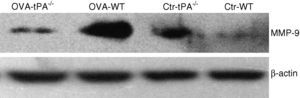
MMP-9 protein contentThe MMP-9 protein content was tested by Western blot assay as described above. The total protein from the nasal mucosa of OVA-challenged and saline-treated mice was extracted. The antibody used was specific for MMP-9.
The Western blot result (Figure 5) was consistent with the relative quantification of MMP-9 mRNA. The amount of MMP-9 protein increased in OVA-challenged WT mice. In the saline-treated mice groups, the t-PA-/- mice expressed more MMP-9 protein compared to the WT mice.
DiscussionThis study provides new insights into the mechanism that t-PA regulates the collagen deposition in an AR mice model. We first showed that depletion of t-PA gene increased collagen deposition in AR nasal mucosa. Secondly, we demonstrated that the expression of MMP-9 decreased in sensitised t-PA gene knockout mice, which showed an opposite expression tendency to u-PA and PAI-1. These suggest that the association of t-PA depletion with MMP-9 expression decrease accounts for the collagen deposition in a t-PA gene knockout AR model.
Studies on the pathology and epidemiology of allergic rhinitis have shown that the remodelling process occurs in allergic rhinitis, epithelial damage and repair, microscopic change in the reticular basement membrane, and blood vessel-related expression of cell growth factors.2 Tissue remodelling usually includes the pathological destruction process and the following tissue reconstruction. Matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinase (TIMP), and transforming growth factor-β (TGF-β), which affect matrix composition synthesis and degradation, play important roles in tissue remodelling.2,12,13 Plasminogen activator, including t-PA and u-PA, are important fibrinolysis proteinases found not only in the cardiovascular system, but also in the tissue matrix.14 The t-PA and u-PA expressions were high in human allergic rhinitis nasal mucosa.9 However, the way in which t-PA affects allergic rhinitis has not yet been directly studied.
In the present study, we inspected the nasal mucosal membrane structure of OVA- and saline(control)-treated t-PA-/- and WT mice. We showed that, after OVA challenge, collagen deposition was more severe in t-PA-/- mice than in WT mice. Moreover, gland hyperplasia was obvious in t-PA-/- mice, and their glandular cells were compressed. There was no obvious structural change in saline-treated controls (data not shown). We believe this was caused by collagen deposition around glands, which hampered the release of secretions.Previous studies showed that t-PA and u-PA are both crucial proteases in matrix remodelling. However, the major plasminogen activator linked to tissue remodelling in different diseases may be various. For example, in endotoxin-treated mice, Yamamoto proved that u-PA activity is linked to fibrin deposition but not t-PA.15 Yang proved disruption of t-PA gene in mice reduces deposition of interstitial collagen III and fibronectin in kidneys in obstructive nephropathy.16 Our previous study showed disruption of t-PA gene in CCl4-treated mice aggravated liver fibrosis by increasing accumulation of ECM and decreasing degradation of ECM.8 PAI-1 is the major plasminogen activator inhibitor in tissue, which showed 20 to 100 fold higher inhibitory activity to t-PA and u-PA than that of PAI-2. Here, we tested u-PA and PAI-1 mRNA expression in an AR model. Results showed that, compared with the saline control, u-PA and PAI-1 mRNA expression increased in both the t-PA-/- and WT AR model. This suggests the nasal mucosa change caused by t-PA gene depletion might not relate with u-PA and PAI-1. However, the increased expression of u-PA in AR nasal mucosa might affect the degradation of ECM in other manners. This might require further investigation.MMPs play an important role in the dynamic equilibrium of extracellular matrix components. Many studies have proven that t-PA modulates the MMP-9 expression and activity which regulates collagen degradation in tissue matrix.8,16 In this study, we showed that after OVA sensitisation and challenge, the MMP-9 mRNA and protein expression increased greatly in WT mice, but MMP-9 expression decreased in t-PA-/- mice. These results suggest that depletion of t-PA decreases collagen degradation in nasal mucosa in AR mice by inhibiting MMP-9 expression.
Sejima et al. believe that t-PA modifies the watery nasal discharge, and that u-PA may help the passage by reducing the viscosity of the discharge in AR.9 In our studies, the depletion of the t-PA gene increased collagen deposition and gland hyperplasia. This observation suggests that enhancing the expression of t-PA in allergic rhinitis might affect the reconstruction of the matrix in the nasal membrane. The lack of t-PA inhibited the expression of MMP-9, an important extracellular matrix-degrading enzyme. The change in MMP-9 expression might play an important function in the reconstruction of the matrix. The pathological function of deposited collagen in allergic rhinitis will be the focus of future investigations.
In conclusion, t-PA gene depletion decreased MMP-9 expression, which may play an important role in the process of increasing collagen deposition in the nasal mucosa matrix.
Conflict of interestThe authors have no conflict of interest to declare.