INTRODUCTION
Since the discovery and characterization 1,2 of immunoglobulin E (IgE) in the mid-sixties, the latter has been regarded as the biological marker for atopic diseases such as rhinoconjunctivitis, bronchial asthma and atopic eczema. In this context, high total serum IgE levels strongly increase the likeliness of atopic disease.
Nevertheless, total serum IgE is also associated with other clinical conditions such as parasitosis, hyper-IgE syndrome and other immunodeficiencies, allergic bronchopulmonary aspergillosis, and certain types of cancer (Hodgkin's disease, IgE myeloma and lung cancer).
The clinical usefulness of IgE assaying as a rapid screening test for allergic diseases has encountered some important obstacles. A first problem is the important overlapping of values between the healthy or non-atopic population and the diseased or sensitized population, which reduces the predictive value of an isolated serum IgE assay 3-5. Secondly, there is great variability in the established serum reference values 6,7, depending on the geographical setting of the study population or even its ethnic origin 8.
This situation makes it necessary to define local reference values for adequate interpretation of the serum IgE findings and these reference values do not always coincide with those specified by the quantitation methodology employed. In an earlier study in 1981 3, we obtained reference values that were significantly higher than those referenced by the PRIST system developed by Pharmacia®9.
In recent decades a genuine increment has been detected in the prevalence of asthma and rhinitis in most countries with a Western lifestyle 10. While on a much more moderate scale, this increase was also observed in our geographical setting, as determined by the data from the Orba Project conducted in the city of Valencia (Spain) and its province 11.
Isolauri 12, in a recent study, reported an increase in the number of cases of biological sensitization to airborne allergens, based on specific IgE determination in different cohorts of individuals born between 1923 and 1990. This observation has been correlated to the increase in the clinical prevalence of asthma.
The present study was designed with two objectives in mind: (a) To update the total serum IgE reference values for the population of the city of Valencia and its metropolitan area using the UniCAP® system from Pharmacia®, with the purpose of comparing them with the reference values established in 1981; and (b) To compare the values obtained with those recorded in the population with bronchial asthma and allergic rhinitis in our setting.
MATERIAL AND METHODS
Serum samples were studied corresponding to two groups of individuals residing in the city of Valencia (Spain) and its metropolitan area. The first group (group D) consisted of 97 blood donors consecutively seen in April 2004 by the mobile extraction team of the Transfusion Center of the Valencian Community. All completed a self-administered questionnaire (Annex 1) similar to that used in 1981, with the purpose of discarding antecedents of atopic disease. Those donors answering any of the items affirmatively were excluded from the study.
The second group (group A) in turn comprised 100 patients examined retrospectively on the basis of a randomized date (September-October 2004) in our Service in correlative order and diagnosed with allergic bronchial asthma with or without allergic rhinoconjunctivitis according to the evaluation criteria normally used in our Service: a compatible clinical history, skin tests, positive specific IgE against allergens of relevance in relation to the established clinical picture, and eventually also allergenic challenge tests in support of the diagnosis.
The samples were collected by venipuncture, and the serum was separated by centrifugation after blood clot retraction at 37 °C. All samples were stored at 18 °C until processing in duplicate with the UniCAP® system from Pharmacia®.
The results were analyzed using the SPSS® version 11.0 statistical package for Microsoft Windows®. The chi-square test was applied for the comparison of proportions, while the Student t-test for independent samples was used for the comparison of means, following verification of normality and homogeneity of variance with the Levene test. A logarithmic-normal model was used for representation purposes.
RESULTS
The donor series comprised 69 individuals aged 20-66 years, after discarding those who had replied affirmatively to some item of the screening questionnaire. The sample with respiratory allergy (asthma and/or rhinitis) consisted of 100 patients with ages in the 13-65 years range.
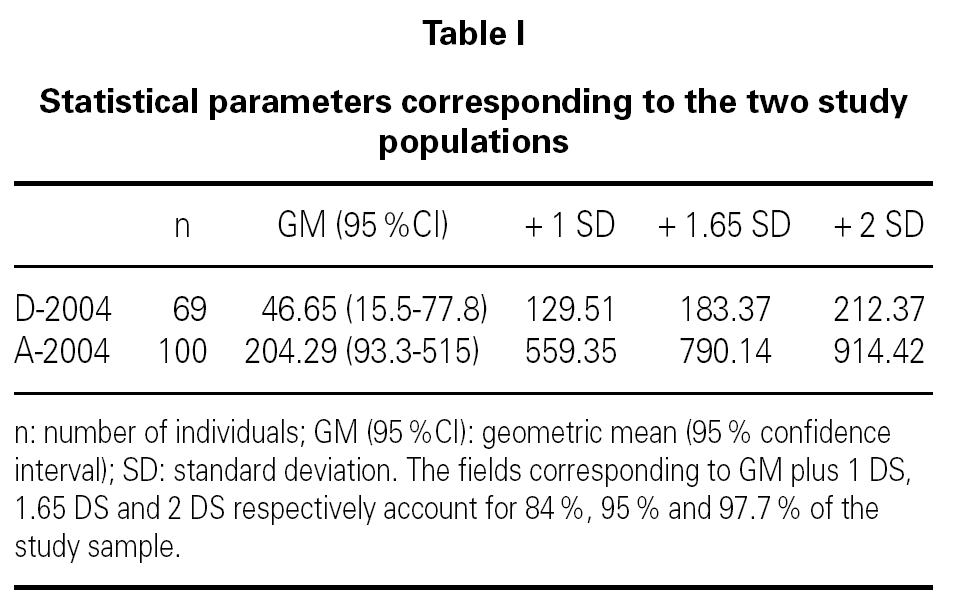
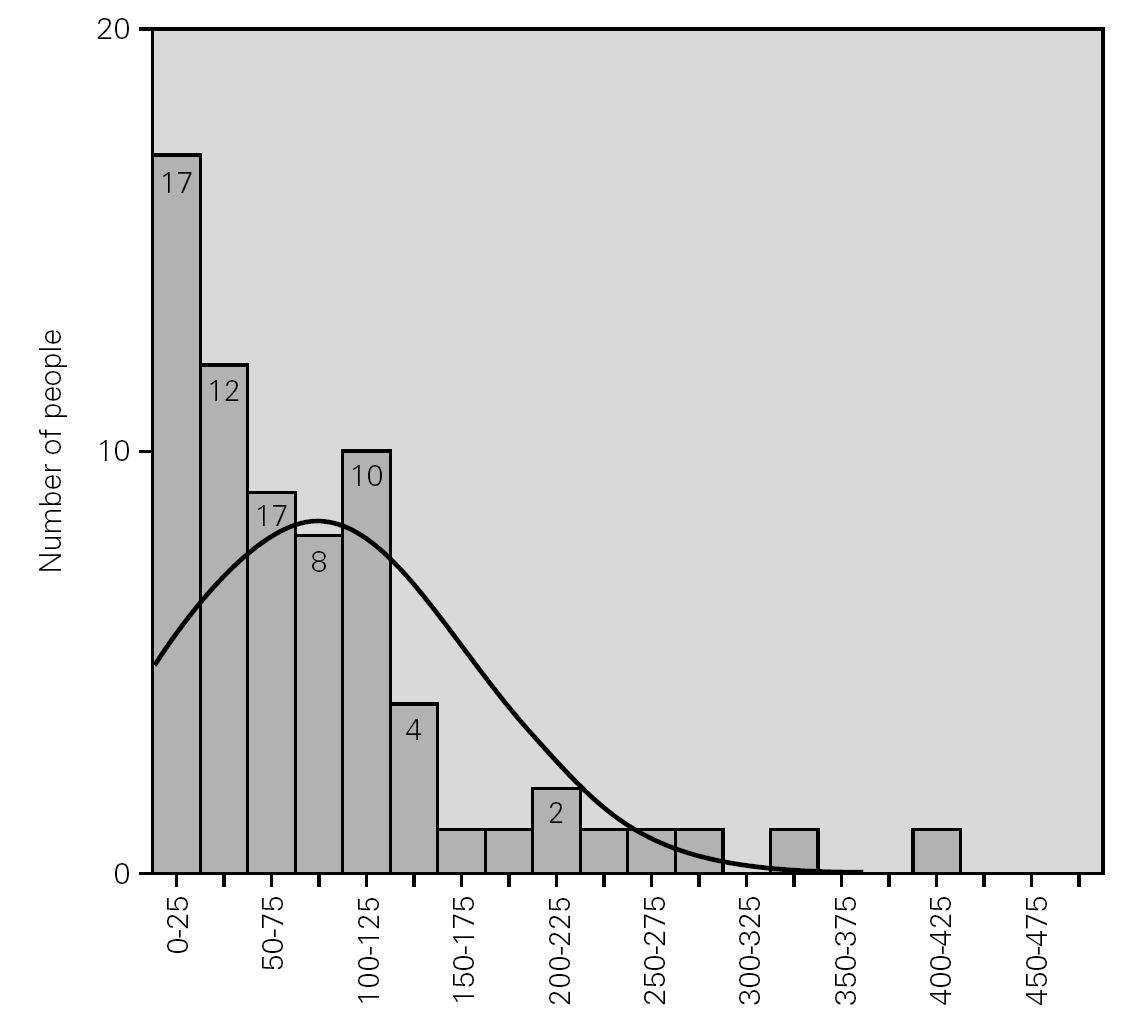
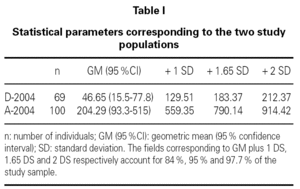
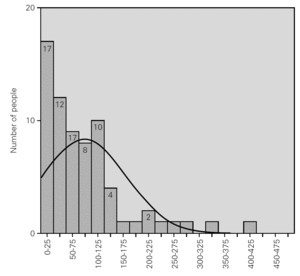
Table I shows the statistical parameters for both samples. Figure 1 in turn reports the frequencies corresponding to the group of donors in 2004.
Figure 1.--Serum level of total IgE in the group of donors.
Comparison of the geometric means of the donors and allergic patients for 2004 proved statistically significant (p < 0.05) thus indicating that the two groups are different. Statistical comparison of the geometric mean of the donors in 2004 versus those in 1981 (geometric mean 29.6 kU/l, with standard deviation (SD) 108 kU/l) was not significant though a tendency towards increased total serum IgE values was noted. However, on comparing with the reference values provided in the instructions manual of the UniCAP® system from Pharmacia® (geometric mean 17.4 kU/l with standard deviation of 47.8 kU/l), the difference in means reached statistical significance (p < 0.05).
On conventionally establishing an upper cut-off point for the group of donors of 183 kU/l (+ 1.65 SD, implying 95 % coverage of the population studied), and applying it to the group of allergic individuals, it is seen that 44 % of the latter exhibit total serum IgE values within the range obtained for the donor population.
For this cut-off point of 183 kU/l, the corresponding sensitivity and specificity is 56 % and 88 %, respectively.
DISCUSSION
The data obtained in the present study confirm the current validness of the total serum IgE reference values obtained in 1981 3.
To our knowledge, no other studies have published reference values for this country with which to establish comparisons. Nevertheless, it may be assumed that our data are more representative of the actual situation in Spain regarding total serum IgE levels than the values supplied in the UniCAP® system instructions, which essentially correspond to the adult Swedish population.
A Dutch study 13 based on a randomly selected series of individuals (not donors) reported total serum IgE values stratified by age and sex with a geometric mean of between 22 and 39 kU/l. The upper values of the "normal" population that covered 95 % of the series were slightly lower (140-162 kU/l) than in our own study.
The overlapping of healthy donors and patients makes individual application of total serum IgE very scantly predictive 3,4.
While the distribution of allergic patients represents a continuum rather than a biphasic curve, to the effects of routine clinical utility it can be assumed that there are two subpopulations of patients with respiratory allergic diseases. One subpopulation presents total serum IgE values similar to those of the "normal" population, i.e., they can be defined as low responders (LRs), while the other subgroup presents total IgE values above those of the donors, and can be referred to as high responders (HRs). In this context, a high total serum IgE value would be indicative of a high probability of respiratory allergy in the absence of other clinical circumstances.
Attempts have been made to obtain different degrees of sensitivity/specificity by varying the corresponding cut-off points 13, though their usefulness in clinical practice is very relative and sometimes clearly discouraging.
We consider our approach to be more realistic in that it assumes the existence of two subtypes of allergic individuals, and postulates that total serum IgE quantitation only serves to detect high responder (HR) allergic individuals. Serum values within the normal range in turn would comprise both healthy subjects and low responder (LR) allergic (or at least sensitized) individuals.
On the basis of such reasoning, it would be interesting to determine the distribution of HRs and LRs among patients with respiratory allergy, their possible distribution according to the culprit antigen and type of pathology involved, and their possible association to the subsequent clinical course of the disease.