Few data are available concerning the time trends and risk factors associated with allergic rhinitis (AR) in schoolchildren in Hungary.
MethodsAt an interval of six years, parents of 6–12-year-old children completed identical ISAAC-based and additional questionnaires related to possible risk factors.
ResultsResponse rate was 62.8% with 6335 questionnaires distributed in 2007, and 52.9% with 6441 questionnaires in 2013. The prevalence of current AR symptoms (subjects presenting clinical symptoms of AR in the past 12 months, but had yet to be diagnosed by physician) increased significantly from 14.9% to 23.5% (p<0.001). There was no significant change in the prevalence of physician-diagnosed AR (11.6–11.2%). In multivariate analysis, gender (OR 0.733; CI 0.642–0.931), a family history of atopy (OR 2.017; CI 1.669–2.436), frequent upper respiratory tract infections (OR 2.033; CI 1.659–2.492), long-lasting disease before the appearance of the allergy (OR 2.119; CI 1.311–3.428), feather bedding (OR 0.773; CI 0.599–0.996) and living in a green area (OR 1.367; CI 1.133–1.650) were found to be significant risk factors of cumulative AR in 2013. In both of the groups with (p<0.000) or without (p<0.003) AR the families with a history of atopy used feather bedding less frequently than families without atopy.
ConclusionAlthough the prevalence of physician-diagnosed AR has not shown significant changes during the studied interval, the significant increase of the current AR symptoms suggests growing prevalence of AR among children in Budapest. Our results revealed new aspects of bedding customs in atopic families.
The ARIA document defines allergic rhinitis (AR) as a symptomatic disorder of the nose, induced after allergen exposure, due to immunoglobulin E-mediated inflammation of the membranes lining the nose.1
AR affects the quality of life of children and incurs medical costs for both the families and the public healthcare system. Comparative time-trend analyses are of paramount importance so that the rapidly changing prevalence of these diseases may be monitored. The International Study of Asthma and Allergies in Childhood (ISAAC), designed as a multicentre-study of the epidemiology of asthma, rhinitis and atopic dermatitis among children, uses standardised questionnaires, which allows doctors to make a possible diagnosis of AR on the basis of patients’ symptoms and allows comparisons worldwide.2,3
Phase I documented over 20-fold variations in the prevalence of self-reported rhinitis symptoms between centres throughout the world for the 13- to 14-years age group. Centres in Argentina (60%, 65%), Paraguay (67%), France (58%) and Brazil (55%) reported the highest 12-month period symptom prevalence of rhinitis in this age group, whereas centres in Ethiopia (3%), India (3–9%) and countries in the former Soviet Union (9%, 10%) recorded the lowest symptom prevalence of AR.4
Phase III found that there was a minimal global increase in the 12-month prevalence of AR symptoms. Prevalence increases in the older children exceeding 1% per year were recorded in 13 centres (3 of 9 centres in Africa, 2 of 15 in Asia-Pacific, 1 of 8 in India, 3 of 15 in Latin America, 3 of 9 in Eastern Europe and 1 of 34 in Western and Northern Europe). Only four centres registered a decrease of a similar scale in rhinoconjunctivitis prevalence.4
The data shows that the prevalence has reached a peak in low and mid-income countries.4 However, few studies in Hungary have addressed this topic. ISAAC Phase III in 2003 registered prevalence of current rhinitis symptoms of 17.8% and 12.9%, respectively, among 13- to 14-year-old and 6- to 7-year-old children in Hungary.5
In 2007, we assessed the prevalence and the risk factors associated with AR symptoms among schoolchildren aged 6–12 years in Budapest.6 The aim of the present study was to re-examine an equivalent age group, with the same questionnaire by the same research team, and to compare the results with those collected six years previously. Prior to our examination only point prevalence studies had been carried out in Hungary. As far as we are aware, this is the first Hungarian study of the time trends in the prevalence of AR among primary schoolchildren. Apart from AR we examined the trends in the prevalence of other allergic diseases such as asthma, eczema and food allergy. By analysing trends, we established whether certain atopic diseases had reached a peak in Hungary. Among other risk factors we analysed the association of feather bedding and atopic family history as this is not considered congruently in the literature.6–10
Material and methodsStudy designTwo cross-sectional studies were performed six years apart (2007 and 2013), in the same 21 randomly selected primary schools in eight districts of Budapest located either in the densely built-up city centre or a leafy suburb. The districts were chosen by simple random sampling by the Central Data Processing and Registration Office of the Hungarian Ministry of Home Affairs. At the initial teacher-parent meetings in September the parents of pupils aged 6–12 years were asked to complete identical ISAAC-based questionnaires, although the studies did not form part of ISAAC.
The numbers of distributed questionnaires in the two years were 6335 and 6441, respectively. Detailed instructions were given by the teachers before completion. The questionnaires were collected immediately after the teacher–parent meetings, or at most a week later. They were completed by the parents of 3933 children in 2007 (response rate: 62.8%); (boys: n=1976; 50.2% and girls: n=1957; 49.8%), and by the parents of 3412 pupils in 2013 (response rate: 52.9%); (boys: n=1637; 48.8% and girls: n=1720; 51.2%).
The study protocol was approved by the Ethics Committee at Heim Pál Children's Hospital Budapest. Informed consent was obtained from all parents. All parents of the patients included in the study received sufficient information.
The questionnaireDetails of the questionnaire, including the definitions, have been published previously.6
The core questions from ISAAC Phase I and its methodology were used.2 The questionnaire was translated into Hungarian. It consisted of two main components: a core questionnaire, which included questions about the symptoms of rhinoconjunctivitis, and an environmental one,11 with questions concerning a variety of potential risk factors. Both cross-sectional studies were carried out in the corresponding season, with identical methodologies, thereby allowing a direct comparison.
The prevalence of “diagnosed AR” was determined on the basis of the answers to the question “Has your child had allergic rhinitis diagnosed by a physician?” The parents of those pupils, who answered yes to this (mentioned) question, did not get questions about current AR symptoms. “Current AR” group consists of pupils who had not been diagnosed with AR by physician, but whose parents gave a positive response to the following question: “In the past 12 months, has your child had a problem with sneezing, or a runny, or a blocked nose when he/she did not have a cold or the flu?” In this way there was no overlap between the two groups. The third question related to allergic rhinoconjunctivitis “In the past 12 months, has this nose problem been accompanied by itchy-watery eyes?” A positive response supports the presence of current AR in comorbidity with allergic conjunctivitis.
The prevalence of “cumulative AR” was taken as the sum of the current AR and the diagnosed AR numbers.
The prevalence of diagnosed allergic disease was determined from the responses to the question “Has your child been diagnosed with an allergic disease by a physician?” If the response was positive, the question, “What kind of allergic disease was it?” identified those pupils who suffered from eczema, food allergy or asthma.
The questions considering the risk factors related to the cumulative AR symptoms can be seen in Table 2.
Statistical analysesAll statistical analyses were performed by a professional statistician using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA). Missing and inconsistent responses were included in the prevalence calculations, but excluded from subsequent bivariate analysis.
The data relating to allergic diseases were characterised with descriptive statistics. The differences between the two time points were explored with T tests, while the Chi-square test and risk-odds ratios (ORs) were used to examine the risk factors for each variable individually with the AR variable. The 95% confidence intervals (95% CI) for the calculated ORs are given in brackets. If a Chi-square test proved significant, the variable was included in the binary logistic regression, in which the AR was the dependent variable. Univariate odds ratio (uOR) computed by crosstabulation examines every single variable versus AR, whereas adjusted odds ratio (aOR) examines those variables in a regression model that significantly affect AR. The results can be seen in the aOR column in Table 2. In Table 2 the cumulative AR n (%) columns show the number and proportion of patients with AR symptoms in the category.
The annual change in prevalence was calculated by taking the difference between the 2007 and 2013 studies’ prevalence values and dividing it by the number of years between the two surveys.
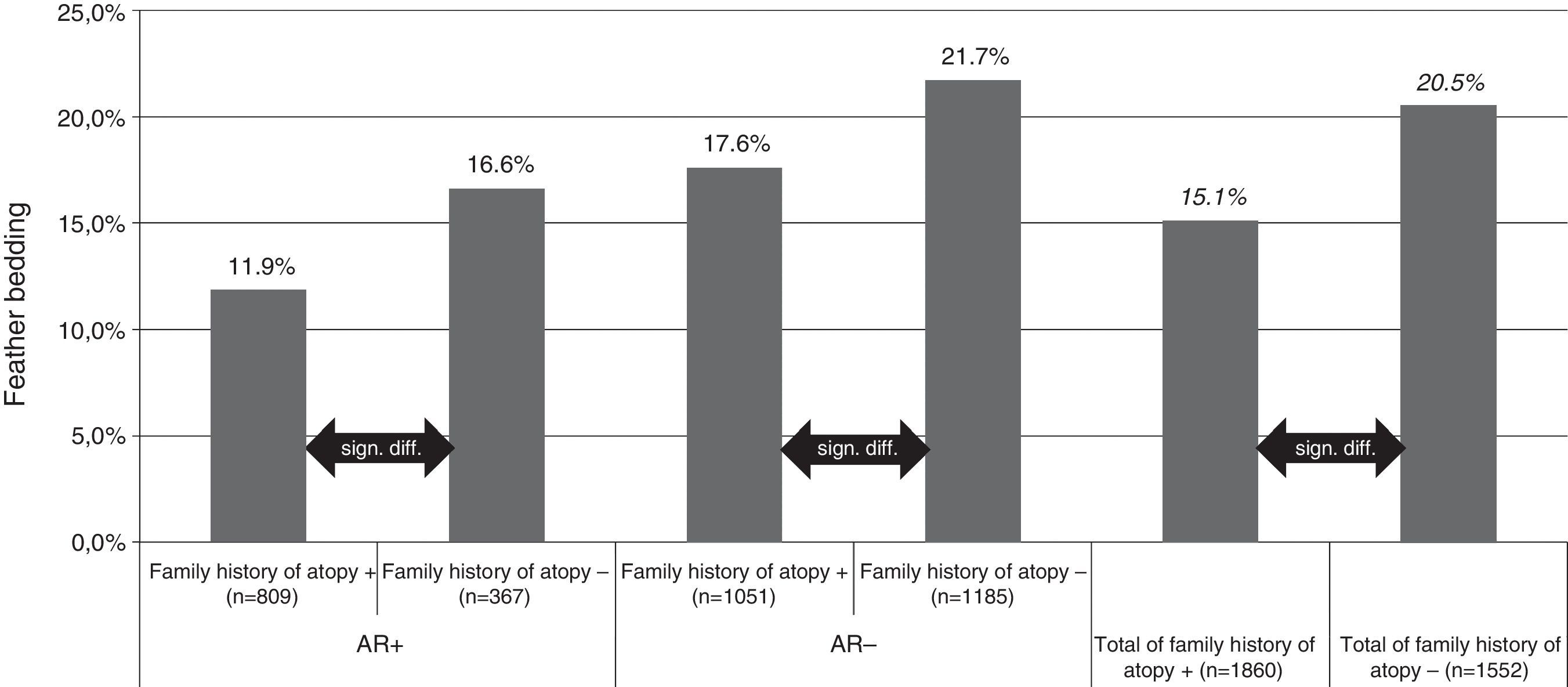
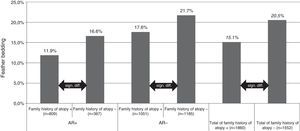
For feather bedding analysis we used T test and ANOVA for the separate four group analysis (Fig. 1). The result was considered statistically significant if p<0.05.
Feather bedding and family history of atopy. AR+: children with allergic rhinitis symptoms; AR−: children without allergic rhinitis symptoms; family history of atopy+: children with family history of atopy; family history of atopy−: children without family history of atopy; n: number of children.
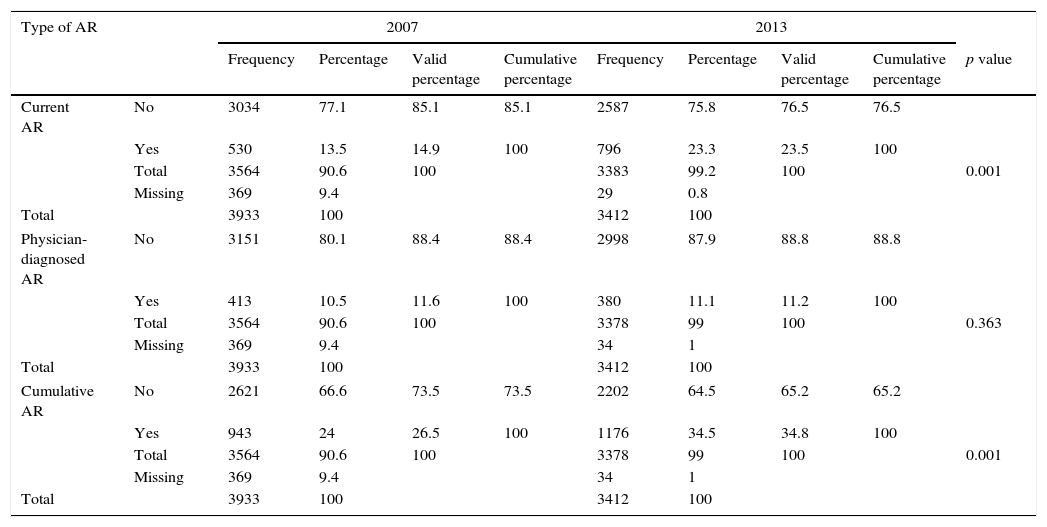
Table 1 demonstrates the trends in the prevalence of current AR, physician-diagnosed AR and cumulative AR in 6–12-year-old children, over the six-year interval from 2007 to 2013. There were significant increases in the prevalence of current AR symptoms, from 14.9% to 23.5% (p<0.001). Prevalence increases exceeding 1% per year (1.43) were recorded. The prevalence increase in the cumulative rhinitis was 1.38% per year (from 26.5% to 34.8%; p<0.001, statistically significant), whereas there was no significant change in the prevalence of physician-diagnosed AR (11.6% and 11.2%).
Prevalence of allergic rhinitis symptoms in children aged 6–12 years in Budapest in 2007 and 2013.
| Type of AR | 2007 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Percentage | Valid percentage | Cumulative percentage | Frequency | Percentage | Valid percentage | Cumulative percentage | p value | ||
| Current AR | No | 3034 | 77.1 | 85.1 | 85.1 | 2587 | 75.8 | 76.5 | 76.5 | |
| Yes | 530 | 13.5 | 14.9 | 100 | 796 | 23.3 | 23.5 | 100 | ||
| Total | 3564 | 90.6 | 100 | 3383 | 99.2 | 100 | 0.001 | |||
| Missing | 369 | 9.4 | 29 | 0.8 | ||||||
| Total | 3933 | 100 | 3412 | 100 | ||||||
| Physician-diagnosed AR | No | 3151 | 80.1 | 88.4 | 88.4 | 2998 | 87.9 | 88.8 | 88.8 | |
| Yes | 413 | 10.5 | 11.6 | 100 | 380 | 11.1 | 11.2 | 100 | ||
| Total | 3564 | 90.6 | 100 | 3378 | 99 | 100 | 0.363 | |||
| Missing | 369 | 9.4 | 34 | 1 | ||||||
| Total | 3933 | 100 | 3412 | 100 | ||||||
| Cumulative AR | No | 2621 | 66.6 | 73.5 | 73.5 | 2202 | 64.5 | 65.2 | 65.2 | |
| Yes | 943 | 24 | 26.5 | 100 | 1176 | 34.5 | 34.8 | 100 | ||
| Total | 3564 | 90.6 | 100 | 3378 | 99 | 100 | 0.001 | |||
| Missing | 369 | 9.4 | 34 | 1 | ||||||
| Total | 3933 | 100 | 3412 | 100 | ||||||
AR: allergic rhinitis.
p<0.05.
The prevalences of comorbid current eye symptoms (itchy eyes and lacrimation) were 81.3% and 93.8% respectively in the current AR group in the corresponding time points (Table 2). The prevalence increase in conjunctivitis was 2% per year, which is the highest rate of all the examined symptoms.
The prevalence of physician-diagnosed asthma remained unchanged from 2007 (6.2%) to 2013 (5.7%). There were significant increases (both at p<0.001) in the prevalence of physician-diagnosed eczema, from 10.2% to 15.4%, and in that of food allergy, from 4.8% to 6.2%.
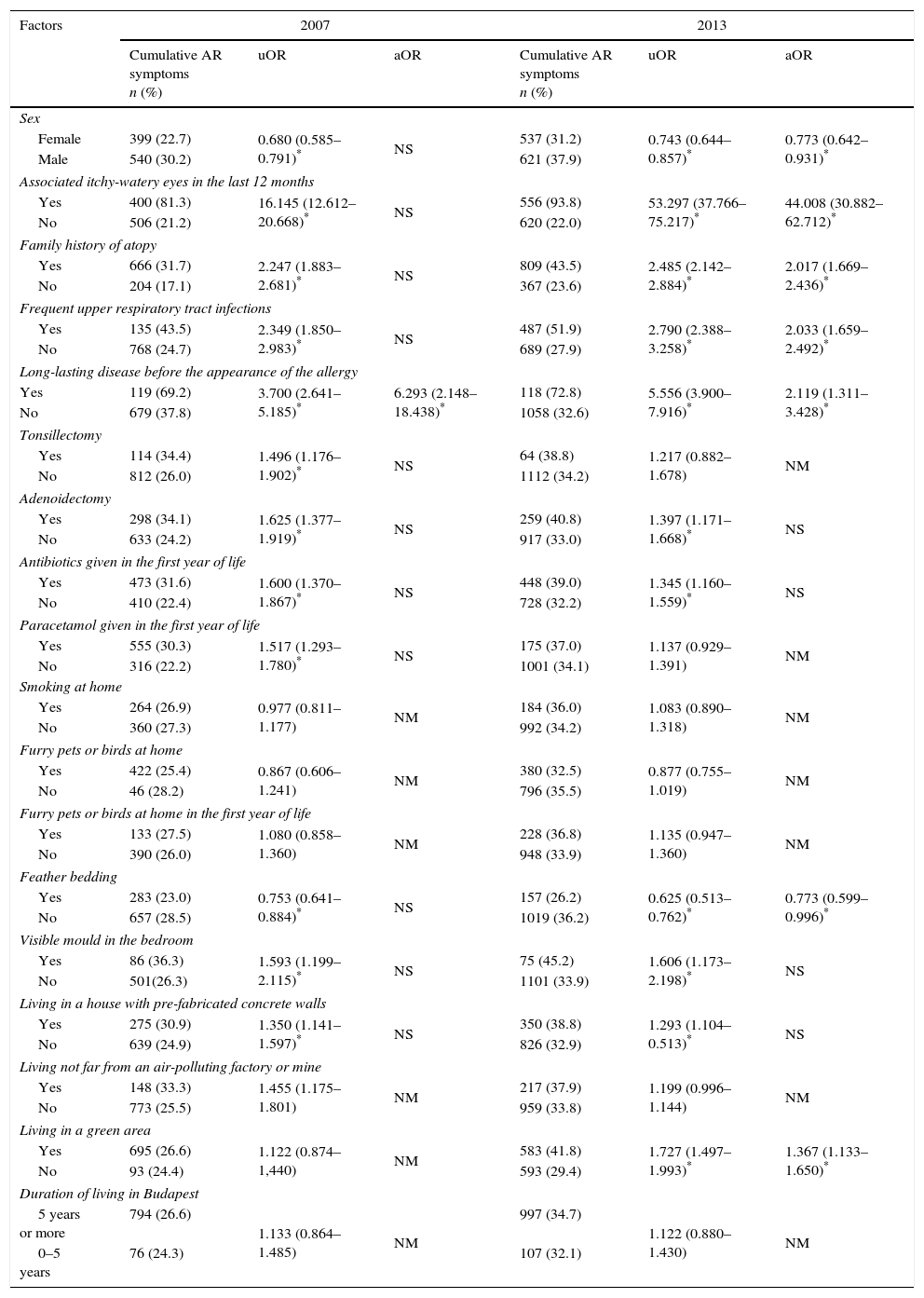
Risk factorsTable 2 presents the findings in 2007 and in 2013 as concerns the relation between the risk factors and the prevalence of cumulative AR.
Factors affecting allergic rhinitis symptoms in 2007 and 2013.
| Factors | 2007 | 2013 | ||||
|---|---|---|---|---|---|---|
| Cumulative AR symptoms n (%) | uOR | aOR | Cumulative AR symptoms n (%) | uOR | aOR | |
| Sex | ||||||
| Female | 399 (22.7) | 0.680 (0.585–0.791)* | NS | 537 (31.2) | 0.743 (0.644–0.857)* | 0.773 (0.642–0.931)* |
| Male | 540 (30.2) | 621 (37.9) | ||||
| Associated itchy-watery eyes in the last 12 months | ||||||
| Yes | 400 (81.3) | 16.145 (12.612–20.668)* | NS | 556 (93.8) | 53.297 (37.766–75.217)* | 44.008 (30.882–62.712)* |
| No | 506 (21.2) | 620 (22.0) | ||||
| Family history of atopy | ||||||
| Yes | 666 (31.7) | 2.247 (1.883–2.681)* | NS | 809 (43.5) | 2.485 (2.142–2.884)* | 2.017 (1.669–2.436)* |
| No | 204 (17.1) | 367 (23.6) | ||||
| Frequent upper respiratory tract infections | ||||||
| Yes | 135 (43.5) | 2.349 (1.850–2.983)* | NS | 487 (51.9) | 2.790 (2.388–3.258)* | 2.033 (1.659–2.492)* |
| No | 768 (24.7) | 689 (27.9) | ||||
| Long-lasting disease before the appearance of the allergy | ||||||
| Yes | 119 (69.2) | 3.700 (2.641–5.185)* | 6.293 (2.148–18.438)* | 118 (72.8) | 5.556 (3.900–7.916)* | 2.119 (1.311–3.428)* |
| No | 679 (37.8) | 1058 (32.6) | ||||
| Tonsillectomy | ||||||
| Yes | 114 (34.4) | 1.496 (1.176–1.902)* | NS | 64 (38.8) | 1.217 (0.882–1.678) | NM |
| No | 812 (26.0) | 1112 (34.2) | ||||
| Adenoidectomy | ||||||
| Yes | 298 (34.1) | 1.625 (1.377–1.919)* | NS | 259 (40.8) | 1.397 (1.171–1.668)* | NS |
| No | 633 (24.2) | 917 (33.0) | ||||
| Antibiotics given in the first year of life | ||||||
| Yes | 473 (31.6) | 1.600 (1.370–1.867)* | NS | 448 (39.0) | 1.345 (1.160–1.559)* | NS |
| No | 410 (22.4) | 728 (32.2) | ||||
| Paracetamol given in the first year of life | ||||||
| Yes | 555 (30.3) | 1.517 (1.293–1.780)* | NS | 175 (37.0) | 1.137 (0.929–1.391) | NM |
| No | 316 (22.2) | 1001 (34.1) | ||||
| Smoking at home | ||||||
| Yes | 264 (26.9) | 0.977 (0.811–1.177) | NM | 184 (36.0) | 1.083 (0.890–1.318) | NM |
| No | 360 (27.3) | 992 (34.2) | ||||
| Furry pets or birds at home | ||||||
| Yes | 422 (25.4) | 0.867 (0.606–1.241) | NM | 380 (32.5) | 0.877 (0.755–1.019) | NM |
| No | 46 (28.2) | 796 (35.5) | ||||
| Furry pets or birds at home in the first year of life | ||||||
| Yes | 133 (27.5) | 1.080 (0.858–1.360) | NM | 228 (36.8) | 1.135 (0.947–1.360) | NM |
| No | 390 (26.0) | 948 (33.9) | ||||
| Feather bedding | ||||||
| Yes | 283 (23.0) | 0.753 (0.641–0.884)* | NS | 157 (26.2) | 0.625 (0.513–0.762)* | 0.773 (0.599–0.996)* |
| No | 657 (28.5) | 1019 (36.2) | ||||
| Visible mould in the bedroom | ||||||
| Yes | 86 (36.3) | 1.593 (1.199–2.115)* | NS | 75 (45.2) | 1.606 (1.173–2.198)* | NS |
| No | 501(26.3) | 1101 (33.9) | ||||
| Living in a house with pre-fabricated concrete walls | ||||||
| Yes | 275 (30.9) | 1.350 (1.141–1.597)* | NS | 350 (38.8) | 1.293 (1.104–0.513)* | NS |
| No | 639 (24.9) | 826 (32.9) | ||||
| Living not far from an air-polluting factory or mine | ||||||
| Yes | 148 (33.3) | 1.455 (1.175–1.801) | NM | 217 (37.9) | 1.199 (0.996–1.144) | NM |
| No | 773 (25.5) | 959 (33.8) | ||||
| Living in a green area | ||||||
| Yes | 695 (26.6) | 1.122 (0.874–1,440) | NM | 583 (41.8) | 1.727 (1.497–1.993)* | 1.367 (1.133–1.650)* |
| No | 93 (24.4) | 593 (29.4) | ||||
| Duration of living in Budapest | ||||||
| 5 years or more | 794 (26.6) | 1.133 (0.864–1.485) | NM | 997 (34.7) | 1.122 (0.880–1.430) | NM |
| 0–5 years | 76 (24.3) | 107 (32.1) | ||||
AR: allergic rhinitis; uOR: univariate odds ratio; aOR: adjusted odds ratio; n: number of children.
In the 2013 study, after the multivariate analysis, the significant risk factors for cumulative AR were male gender, a family history of atopy, frequent upper respiratory tract infections, long-lasting disease before the appearance of the allergy and living in a green area.
In the 2007 study, in the multivariate analysis, only long-lasting disease before the appearance of the allergy proved to be such a risk factor.
Both studies with the univariate analysis and the multivariate analysis in 2013 revealed that the odds ratio was smaller for AR prevalence if the pupils were exposed to feather bedding. Feather bedding was significantly lower in children with a family history of atopy (15.1%, p<0.0001) compared with the group where the family history was negative for atopy (20.5%, p<0.0001). Interestingly, the significant difference in feather bedding between atopic or non-atopic families could be observed in both, the subject group with (11.9% versus 16.6%, p<0.000) or without (17.6% versus 21.7%, p<0.003) AR symptoms (Fig. 1).
DiscussionRepeated cross-sectional studies over an adequate period of time, on a given population, with identical study methods, permit a reliable assessment of the trend of AR prevalence. Our results demonstrate that the prevalence of cumulative AR and current AR symptoms (AR in the past 12 months) in 6–12-year-old children increased significantly (from 26.5% to 34.8% and from 14.9% to 23.5%, respectively) in the six-year interval analysed.
Several time-trend prevalence studies have reported that the incidence of AR among primary schoolchildren has recently been increasing.12,13 Our findings document that AR affected more than a third of the 6–12-year-old children in Budapest in 2013. ISAAC Phase III14 found that the global prevalence for current AR in the 13–14-year-old group was 14.1%, and in the 6–7-year-old group was 8.5%. In comparison, the prevalence of current AR symptoms among primary schoolchildren aged 6–12 years in 2013 was much higher (23.5%).
81.3% of the children with current AR had conjunctivitis in 2007. This figure increased to 93.8% in 2013. The very high proportion of patients having conjunctivitis may suggest comorbidity. It also suggests that parents are able to make a difference between infectious rhinitis and allergy. Our data can be compared with the international trends.4
The prevalence of diagnosed AR (11.6% and 11.2%) did not change in the given interval. We presume that this is possible partly because of the changes in the healthcare system. At the time of our first study, in 2007 only ENT or allergy and immunology specialists were allowed to prescribe subsidised medicine for AR following a thorough examination of the patient, or to suggest that GPs should prescribe it. By the time of the second study, GPs were able to prescribe medicine without a specialist's diagnosis. On the other hand, anti-allergic medicines are now available over-the-counter. As a result, self-diagnosis and self-treatment are on the rise.
No significant difference was found in the prevalence of asthma (6.2–5.7%).
The prevalence of diagnosed eczema (from 10.2% to 15.4%) and diagnosed food allergy (from 4.8% to 6.2%) increased significantly, which may possibly be explained by genetic factors, such as a family history of atopy, and by rapid environmental changes.
Risk factorsOur findings are in line with those of previous studies.4,15,16 It seems that male gender may be considered a risk factor for the development of AR in primary school children, although the underlying causes are unknown.
As in several other studies,11,17 a family history of atopy correlated significantly with the prevalence of cumulative AR. Genetic susceptibility enhances the risk of the appearance of allergic diseases.
Frequent upper respiratory tract infections significantly increased the prevalence of cumulative AR. Experimental research results18 support the epidemiological data17 that viral respiratory infection may contribute to allergic sensitisation.
The results of both of our studies revealed that long-lasting disease before the appearance of the allergy significantly increased the risk of the development of cumulative AR. Children have six to eight viral upper respiratory infections per year.19 These infections may be complicated by secondary bacterial infection, and the respiratory tract may be considered a unique morphofunctional entity.20
Both of our studies suggested that the prevalence of cumulative AR was significantly higher among pupils who underwent adenoidectomy, although logistic regression analysis did not indicate this as a significant factor explaining the presence of the AR. Some recent publications21,22 concluded that the presence of AR leads to allergic inflammation around the adenoid tissue, thereby increasing the possibility of adenoid hypertrophy. Statistical analyses demonstrated that the occurrence of adenoid hypertrophy in children with AR peaked in the group of 6-year-olds.21 Our study population (6–12-year-old schoolchildren) may have had problems leading to adenoidectomy before the allergy was diagnosed by a physician.
Both of our studies revealed that the administration of antibiotics given in the first year of life is significantly associated with the development of cumulative AR, as previously demonstrated by the largest study of its type involving the participation of 193,412 children from 71 centres in 29 countries.23 As suggested by the hygiene hypothesis, antibiotic use during infancy can increase the risk of atopic diseases by decreasing the protective effect of the gut microbiotic flora.16
In the studies by Siebers at al., feather pillows were found to harbour lower levels of house dust mite allergens than non-feather pillows, the reason perhaps being that the tighter weave of the pillow cover makes it more impermeable to house-dust mites.7 At first sight, feather bedding may seem to be a risk factor but in our population we found that family history of atopy lessens the habit of feather bedding compared to non-atopic families (15.1% versus 20.5%). In 2007, 23.0%, and in 2013, 26.2% of the children exposed to feather bedding had symptoms of AR as compared with 28.5% in 2007 and 36.2% in 2013 of the pupils not exposed to feather bedding. Our data are in line with other epidemiological studies, but our results add a new question to the epidemiological evidence of bedding and atopic family history: that avoidance of feather bedding could be a result of an atopic family's choice.8–10 It is possible that being aware of the advantages of the avoidance of indoor allergens, the parents of atopic children may have decided not to use feather bedding. Our epidemiological examination measured a smaller feather bedding ratio among patients with a family history of atopy with AR, as well as without AR (Fig. 1).
Visible mould in the bedroom significantly increased the risk of the development of cumulative AR in both of our analyses, supporting the data of a number of epidemiological studies.11,24,25 Home dampness promotes the growth of fungi, their spores and toxins. Home dampness and mould exposure have been associated with the development of allergic diseases.26
In both of our studies, the risk of the development of AR was significantly higher in children who lived in a house with prefabricated concrete walls. Newly built or renovated houses with insulated windows and central heating systems have been reported to have higher levels of indoor air pollutants, chemical products used for cleaning and cockroach allergens.24,27 Our 2013 study demonstrated a positive correlation between living in a green area (leafy suburbs) and cumulative AR. In the green areas of Budapest, there are more grasses, trees and weed species than in other parts. Some studies have suggested that environmental pollution enhances the allergenicity of pollen.28 By attaching to the surface of pollen grains and of plant-derived paucimicronic particles, air pollutants can modify the morphology of these antigen-carrying agents and change their allergenic potential. Consequently, air pollution may not only worsen the symptoms of AR, but may also promote airway sensitisation to airborne allergens in susceptible patients.29
Limitations and strengthsSome limitations should be considered when interpreting our results. The prevalence of AR was measured based on questionnaires, without performing clinical examinations or allergy skin tests to confirm reported symptoms. Current AR symptoms were related to the past 12 months, which may involve further limitations of the study. On the other hand, the reported “itchy-watery eyes” concurrently with the nasal symptoms give support to “current AR”. Thus, the outcomes may have been over- or underestimated. The strength of this study is the comparability of the results. The authors used a standardised method duplicated in a repeat survey in identical schools, identical age groups, surveyed six years apart. In addition, the data set is large enough to reflect the population of Budapest.
ConclusionThe prevalence of current AR, cumulative AR, diagnosed eczema and diagnosed food allergy in a 6–12-year-old population in Budapest has clearly continued to display an increasing trend, while the prevalence of diagnosed AR and asthma has remained unchanged. Children who manifested long-lasting disease before the appearance of the allergy had a 6.3-fold and a 5.5-fold risk of AR in the two examined years. Our data suggest that the awareness of the parents of atopic children results in their decision to reduce the exposure of their children to feather bedding. Our study has not verified that feather bedding would be a protective factor as far as rhinitis is concerned, but it has proven the fact that the atopic families tend to use feather bedding significantly less frequently.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Funding sourceNone.
Authors’ contributionAll authors participated: (1) in the conception and design of the study, acquisition of data, analysis and interpretation of data; (2) in drafting the article and revising it critically for important intellectual content; (3) in final approval of the version to be submitted.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors wish to thank the pupils, their parents and the heads, teachers and secretaries of the schools for their help and co-operation during the data collection phase. They are also grateful to the librarian at Heim Pál Hospital for Sick Children in Budapest for the reference retrievals.