Functional and inflammatory measures have been recommended to corroborate asthma diagnosis in schoolchildren, but the evidence in this regard is conflicting. We aimed to determine, in real-life clinical situation, the value of spirometry, spirometric bronchial reversibility to salbutamol (BDR), bronchial responsiveness to methacholine (MCT) and fractional exhaled nitric oxide (FENO), to corroborate the diagnosis of asthma in children on regular inhaled corticosteroids (ICS) referred from primary care.
MethodsOne hundred and seventy-seven schoolchildren with mild-moderate persistent asthma, on treatment with regular ICS, participated in the study. Abnormal tests were defined as FENO ≥ 27 ppb, BDR (FEV1 ≥ 12%) and methacholine PC20 ≤ 4 mg/mL.
ResultsThe proportions of positive BDR, FENO and MCT, were 16.4%, 33.3%, and 87.0%, respectively. MCT was associated with FENO (p < 0.03) and BDR (p = 0.001); FENO was associated with BDR (p = 0.045), family history of asthma (p = 0.003) and use of asthma medication in the first two years of life (p = 0.004). BDR was significantly related with passive tobacco exposure (p = 0.003).
ConclusionsSpirometry, BDR and BDR had a poor performance for corroborating diagnosis in our asthmatic children on ICS treatment; on the contrary, MCT was positive in most of them, which agrees with previous reports. Although asthma tests are useful to corroborate asthma when positive, clinical diagnosis remains the best current approach for asthma diagnosis, at least while better objective and feasible measurements at the daily practice are available. At present, these tests may have a better role for assessing the management and progression of the condition.
Asthma is a highly prevalent condition in children causing an elevated use of health facilities, large expenses in medications and usually impairing the quality of life of patients and their families.1 At the daily medical practice, the diagnosis of asthma in children is mainly based on the clinical history and physical examination; however, the major features of asthma i.e., reversible airway obstruction, bronchial hyperresponsiveness (BHR) and airway inflammation, can be measured using relatively simple tests. Thus, spirometry and bronchodilator response (BDR), assessment of bronchial responsiveness, and measurements of the fraction of exhaled nitric oxide (FENO), together with some diagnostic algorithms, have been recommended to establish or corroborate asthma diagnosis in children;2–4 nonetheless, the performance of those asthma tests and algorithms would be low.4–9 Furthermore, the information on the diagnostic value of asthma tests in children is seldom collected under real-life situations, i.e., referred patients on different stages of asthma control, different treatments and levels of adherence to treatment, inadequate inhalation techniques, varying severity classifications, among others. At present, most asthmatic children referred to respiratory specialists for uncontrolled asthma, further study, or simply to corroborate the diagnosis, are on treatment with inhaled corticosteroids (ICS). It has been shown that ICS can reduce asthma symptoms, BHR, BDR and FENO levels to different extents,10,11 which could make the interpretation of those tests in asthmatic children on treatment with ICS difficult.
This study was undertaken to determine the performance of commonly used tests for asthma diagnosis i.e., spirometry, BDR to salbutamol, methacholine challenge test (MCT) and FENO, to corroborate asthma diagnosis in asthmatic children on ICS treatment referred from primary care.
Material and methodsThis was a cross-sectional observational study in which we consecutively studied 177 non-smoking children (aged 7–14 years) with mild-moderate persistent asthma. Patients were sent to our paediatric respiratory clinic from primary care to corroborate asthma diagnosis because of persistent asthma symptoms despite treatment with regular ICS, or doubtful asthma diagnosis. At entry, the diagnosis of asthma was confirmed by the study’s paediatric respiratory physicians according to the clinical history as given by parents (recurrent wheezing, cough, chest tightness, among others) and physical examination. Then the patients were scheduled for performing FENO, spirometry and BDR, MCT; all tests were completed within five days after entry to study and following the same order. Children were included in the protocol if they had been free of asthma exacerbation, lower respiratory tract infections and use of systemic corticosteroids in the last four weeks. Patients whose parents reported co-morbidities able to cause respiratory repercussions (cardiopathies, cystic fibrosis, etc.) did not participate in the study. Salbutamol was discontinued for 12 h before testing and ICSs were maintained according to prescription from primary care; none of the participating children were using oral anti-histamines, long-acting beta two agonists, anti-leukotrienes or theophylline.
On-line single breath FENO measurements (NIOX MINO, Aerocrine AB, Solna, Sweden) were performed according to ATS guidelines,12 and the detailed procedure has been previously reported.10 FENO ≥ 27 ppb was taken as a positive test according to our previous findings in healthy children living in the same area as the patients in the present study.13 Some guidelines have suggested using data from the local heathy population to determine cut-off values for FENO and suggested a value of ≥35 ppb for defining and abnormal FENO in children.12
Spirometry was performed using a pre-Vent flow sensor with the Medgraphics CPFS/D processing system (Medical Graphics Corp.; St Paul, Minnesota, USA). The percentage of predicted value for each parameter (FVC, FEV1, and FEV1/FVC) was calculated according to Knudson’s equations14 and abnormal values were defined as less than the fifth percentile. Positive BDR was defined as ≥12% increase from baseline in FEV1 measured 15 min after inhaling 400 µg of salbutamol,4,15 administered via metered dose inhaler with spacer.
MCT test was performed if FEV1 was >80% of predicted value. Following inhalation of normal saline, doubling concentrations of methacholine from 0.03 mg/mL to 8 mg/mL were inhaled every five minutes until reaching a FEV1 fall greater than 20 % of the post-saline value. The provocative concentration of methacholine resulting in a 20% fall of FEV1 (PC20) was calculated by linear interpolation;16 a positive MCT was defined as a PC20 ≤ 4 mg/mL.17
Skin prick test (SPT) for eight common inhalant allergens was performed on the forearm, plus positive (histamine) and negative (solvent) control. The following allergens were employed: Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, alternaria, a grass mixture, a tree mixture and a weed mixture (Nelco Laboratories, NY, US). Atopy was defined as a positive reaction (wheal size measuring ≥3 mm after subtraction of the negative control) to any of the tested allergens.
This study was approved by the Scientific Ethics Committee, Chilean Ministry of Health, Southern Metropolitan Area of Santiago de Chile. Full informed and signed consent was obtained from all parents.
Data analysisThe proportions of patients who had positive FENO, BDR and MCT were analysed using descriptive statistics and variables showing non-normal distribution were log transformed. Correlation among positive tests was estimated by the Spearman test and differences between proportions for each positive test according to potential risk factors for asthma as sex, atopy, family history of asthma, tobacco during pregnancy, passive tobacco exposure at home, recurrent wheezing and use of inhaled asthma medication in the first two years of life, were assessed using non-parametric tests.
Apart from the proportion of children with positive tests according to the cut-offs used in this study for FENO (≥27 ppb), BDR (ΔFEV1 ≥ 12%) and MCT (PC20 ≤4 mg/mL), the proportions of patients with positive tests resulting when using other reported cut-offs for FEV1 (8%),6 FENO (≥35 ppb),12 PC20 (2.5 mg/mL),18 and FEF25-75% (≥30%),19 were also described for illustration.
Regression analysis was employed for studying associations among continuous variables as FENO (ppb), PC20 (mg/mL) and BDR (% of FEV1 change after salbutamol); a value of p < 0.05 was considered as statistically significant. Data were analysed using statistical software (MedCalc v19.0.3, Ostend, Belgium).
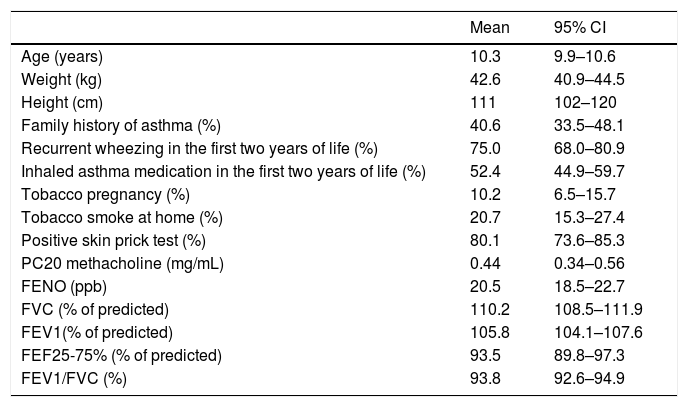
ResultsOf the 180 asthmatic children initially included in the study, 177 (96 boys and 81 girls) completed all study measurements; their mean age, height and weight was 10.3 ± 2.2 years, 142.5 ± 16.3 cm and 44.3 ± 12.7 kg, respectively; other relevant data and baseline measurements of lung function and FENO are summarised in Table 1. At entry and before performing spirometry, BDR, MCT and FENO, the clinical diagnosis of asthma was established by the study’s respiratory physicians in all children and study tests were completed in the first five days after entry.
Characteristics of patients and related variables.
| Mean | 95% CI | |
|---|---|---|
| Age (years) | 10.3 | 9.9–10.6 |
| Weight (kg) | 42.6 | 40.9–44.5 |
| Height (cm) | 111 | 102–120 |
| Family history of asthma (%) | 40.6 | 33.5–48.1 |
| Recurrent wheezing in the first two years of life (%) | 75.0 | 68.0–80.9 |
| Inhaled asthma medication in the first two years of life (%) | 52.4 | 44.9–59.7 |
| Tobacco pregnancy (%) | 10.2 | 6.5–15.7 |
| Tobacco smoke at home (%) | 20.7 | 15.3–27.4 |
| Positive skin prick test (%) | 80.1 | 73.6–85.3 |
| PC20 methacholine (mg/mL) | 0.44 | 0.34–0.56 |
| FENO (ppb) | 20.5 | 18.5–22.7 |
| FVC (% of predicted) | 110.2 | 108.5–111.9 |
| FEV1(% of predicted) | 105.8 | 104.1–107.6 |
| FEF25-75% (% of predicted) | 93.5 | 89.8–97.3 |
| FEV1/FVC (%) | 93.8 | 92.6–94.9 |
RW, recurrent wheezing (≥3 wheezing episodes diagnosed by physician); ppb, parts per billion.
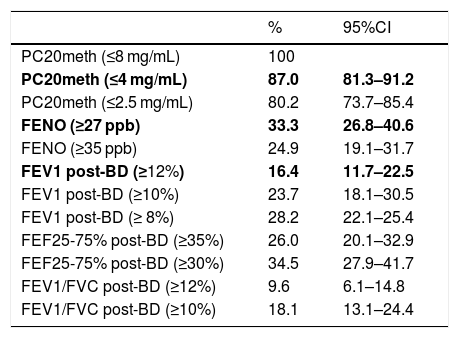
Using the study cut-offs for defining positive tests, the proportion of children who had at least one positive test was 89.3% (95%CI 83.8–93.0), mainly explained by the high proportion of children with positive MCT (87%). When considering just FENO and BDR, a 49.7% (95%CI 42.4–57.0) of patients had one of those tests positive, and 6.8% of children had both FENO and BRD positive. The proportions of asthmatic children with positive tests that occurred if we had used other previously reported cut-offs for FENO,12 BDR6 and MCT,18 or for other spirometric parameters as FEF25-75%19 and VEF1/FVC, are displayed for illustration in Table 2.
Proportion (%) of children diagnosed as asthmatics on clinical bases who had at least one of the following functional or inflammatory indicators positive. Study cut-offs are in bold.
| % | 95%CI | |
|---|---|---|
| PC20meth (≤8 mg/mL) | 100 | |
| PC20meth (≤4 mg/mL) | 87.0 | 81.3–91.2 |
| PC20meth (≤2.5 mg/mL) | 80.2 | 73.7–85.4 |
| FENO (≥27 ppb) | 33.3 | 26.8–40.6 |
| FENO (≥35 ppb) | 24.9 | 19.1–31.7 |
| FEV1 post-BD (≥12%) | 16.4 | 11.7–22.5 |
| FEV1 post-BD (≥10%) | 23.7 | 18.1–30.5 |
| FEV1 post-BD (≥ 8%) | 28.2 | 22.1–25.4 |
| FEF25-75% post-BD (≥35%) | 26.0 | 20.1–32.9 |
| FEF25-75% post-BD (≥30%) | 34.5 | 27.9–41.7 |
| FEV1/FVC post-BD (≥12%) | 9.6 | 6.1–14.8 |
| FEV1/FVC post-BD (≥10%) | 18.1 | 13.1–24.4 |
PC20meth, PC20 methacholine; Post-BD, % of change after 400mcg of salbutamol.
Positive SPT was present in 80.1% (95% CI 73.6–85.3) of patients. Atopic asthmatic children had a significantly higher proportion of positive FENO (41.1%) than non-atopic asthmatics (2.9%) (p < 0.0001). There was no significant difference between atopic and non-atopic asthmatics regarding positive MCT (p = 0.59) or BDR (p = 0.49). It is worth noting that 58.9% (95%IC 50.6–66.7) of the atopic asthmatic children had normal FENO. Family history of asthma was significantly associated with positive FENO (p = 0.003) but not to positive MCT (p = 0.27) or BDR (p = 0.53), whereas the use of inhalers for asthma in the first two years of life was significantly associated with positive FENO (p = 0.004) but not to MCT (p = 0.66) or BDR (p = 0.84). Tobacco during pregnancy was not associated to positive FENO, PC20 or BDR, whereas positive ID tobacco exposure was significantly related with positive BDR (p = 0.003) but not to positive FENO or MCT. The proportion of asthmatic children with positive MCT that showed positive BDR and positive FENO was 18.8% (95%CI 13.44–25.74) and 35.7% (95%CI 28.58–43.55), respectively. The correlation among positive tests (FENO, BDR and MCT) was mostly non-significant, excepting for a weak albeit significant correlation between positive MTC and BDR (p = 0.017).
The results of the non-parametric analysis of variance including methacholine PC20 (mg/mL), FENO (ppb) and BDR (% of change) as continuous variables and asthma-related factors as categorical variables showed that FENO (ppb) was significantly higher in children with family history of asthma (p = 0.001), inhaled asthma medications during the first two years (p = 0.034), and atopy (p < 0.001). Methacholine PC20 (mg/mL) was significantly lower only in children with a family history of asthma (p = 0.07); whereas BDR (% of FEV1 change) was higher in children exposed to ID tobacco (p < 0.0001).
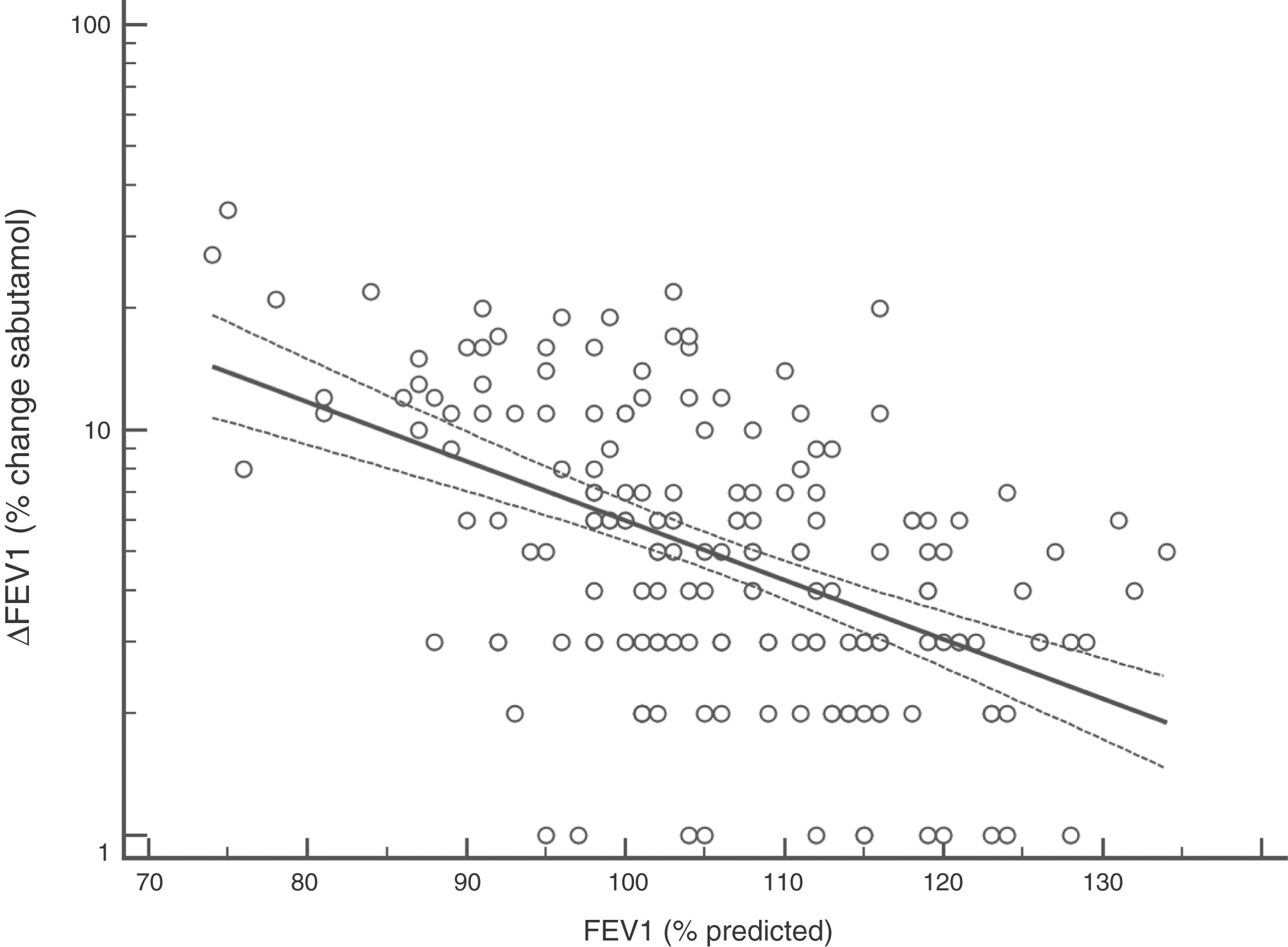
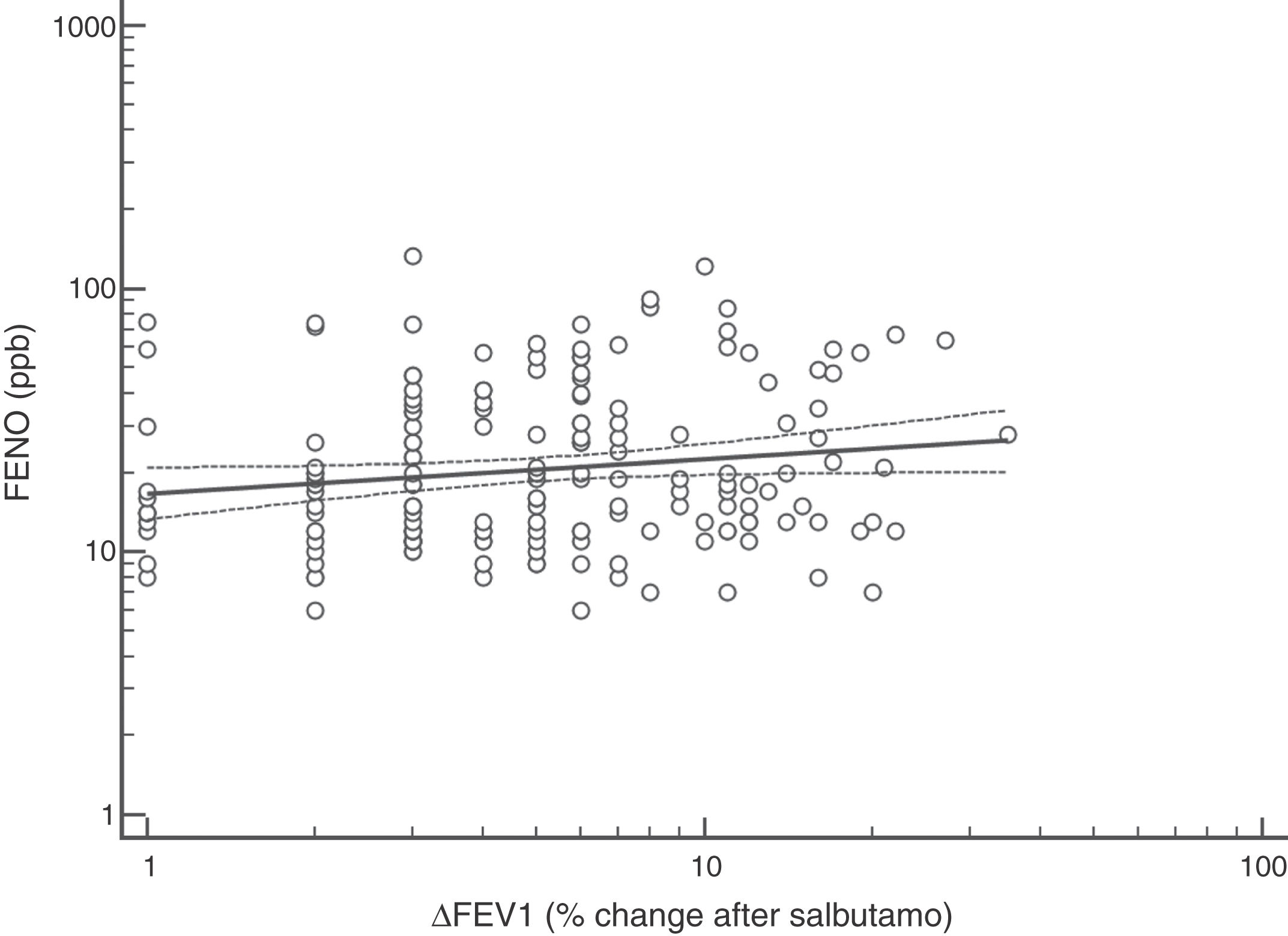
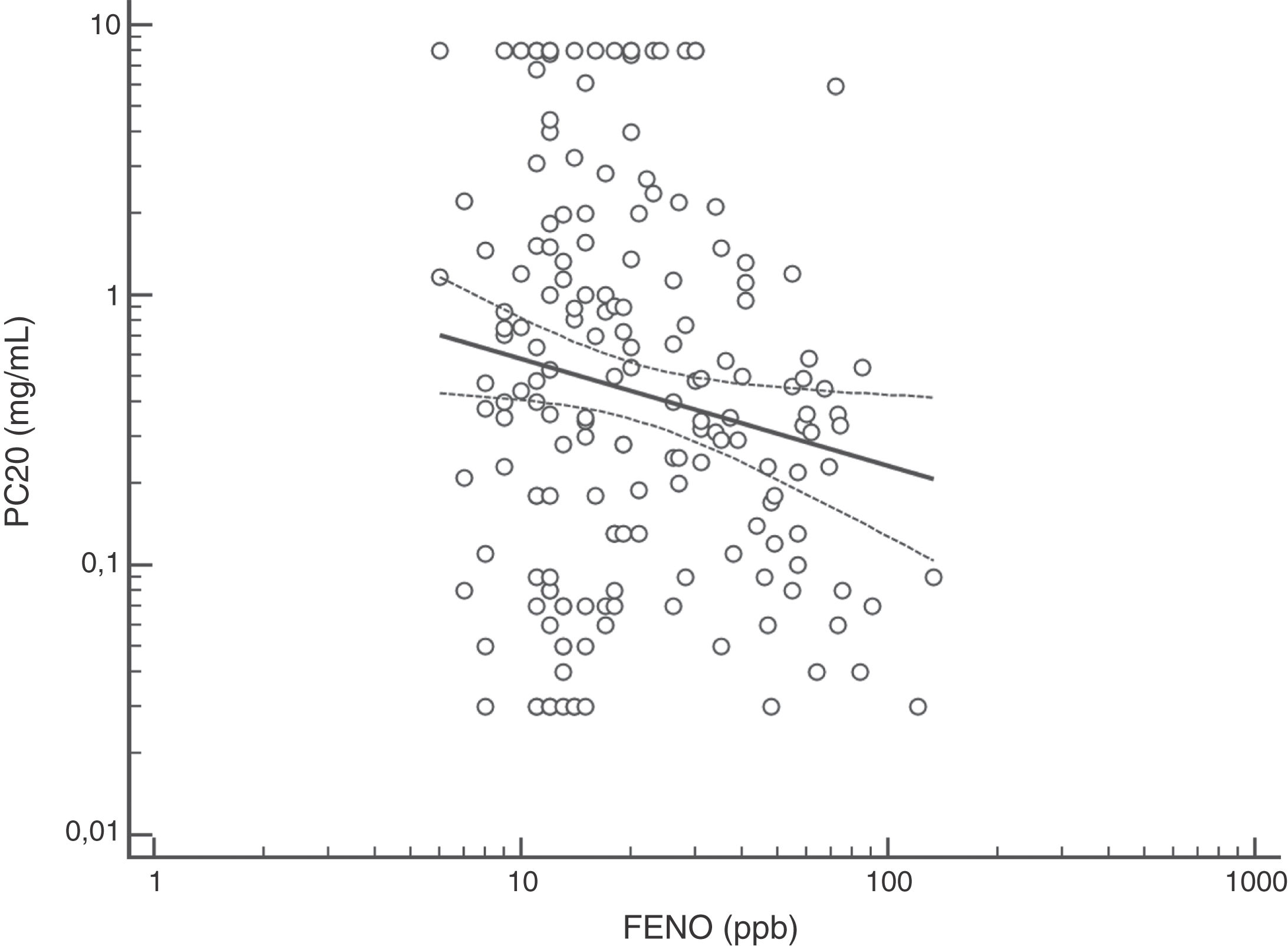
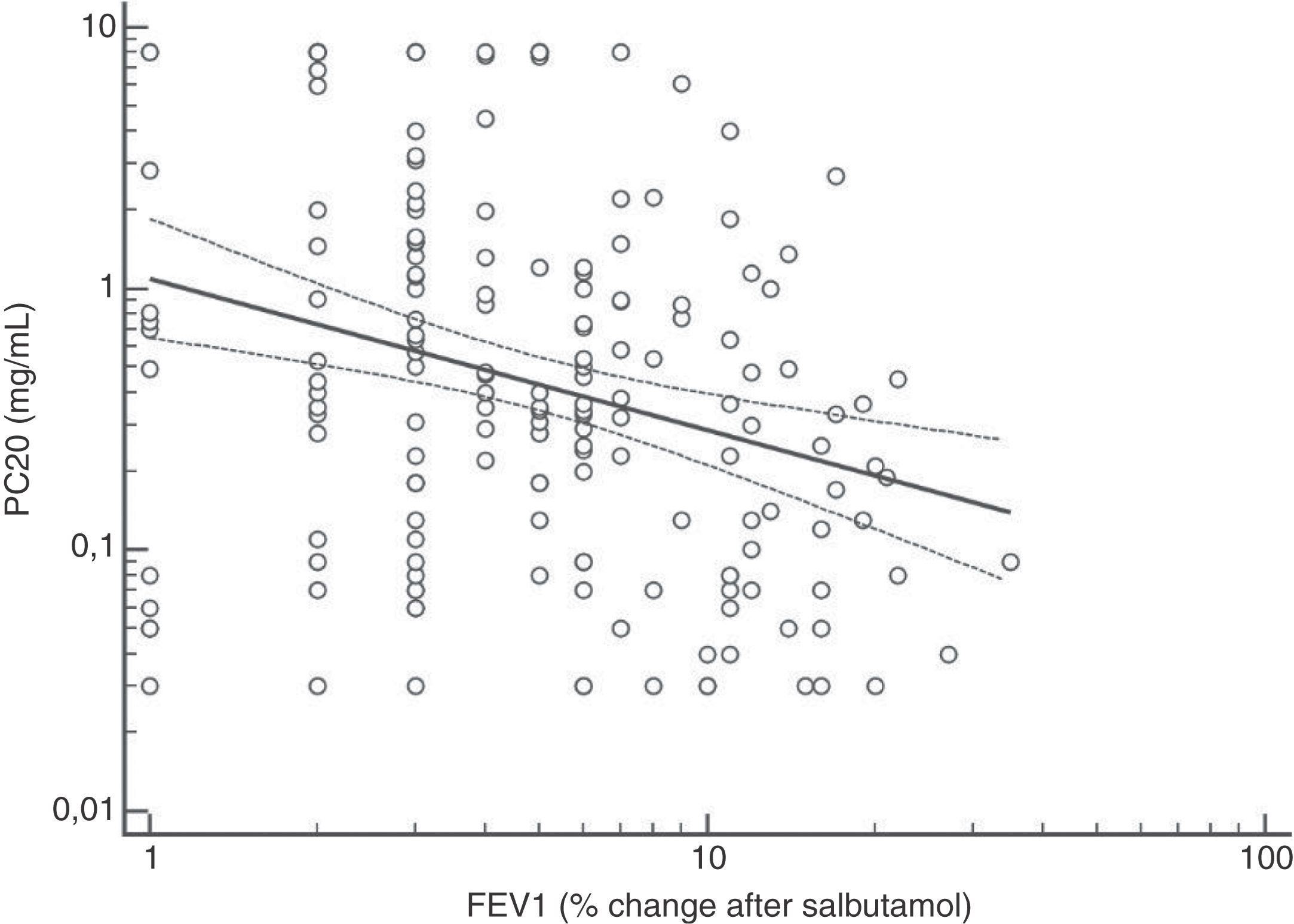
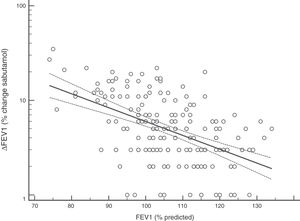
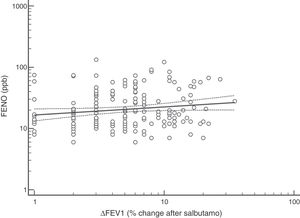
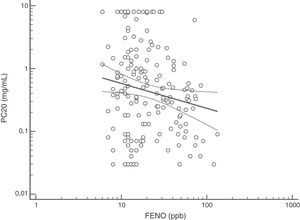
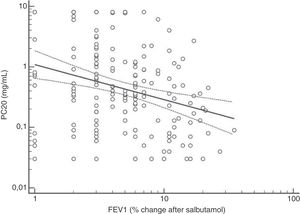
FENO (ppb) showed a weak albeit significant association with BDR (p = 0.046); a significant relationship was found between FEV1 (% predicted) and BDR (% of change), p < 0.0001, between methacholine PC20 (mg/mL) versus FENO (ppb) (p = 0.027) and methacholine PC20 (mg/mL) versus BDR (p < 0.001), Figs. 1–4.
This study shows that spirometry, BDR and FENO would have a poor performance to corroborate asthma diagnosis in children with mild-moderate persistent asthma on treatment with ICS. Just 33.3% of patients when using FENO, and 16.4% if using BDR, had been corroborated as asthmatics. On the other hand, MCT at 4 mg/mL PC20 cut-off was positive in a very high proportion of the children clinically diagnosed as asthmatics by the paediatric respiratory physicians, at entry. Our findings add to those previously reported with respect to the low performance of baseline spirometry, FENO and BDR, among others, to corroborate asthma diagnosis or the level of asthma control, with an additional low correlation among asthma positive tests.5–11
The high proportion of asthmatic children on ICS with positive MCT agrees with previous studies using different cut-off for BHR.16,18,20–25 Despite methodological and interpretative considerations related to MCT (and other challenge tests) for the diagnosis of asthma in adults and children,17,18,20–27 this test would be useful when studying, managing and following up patients with difficult to control or persistent asthma symptoms despite ICS treatment, as found in this study. At present, there is consensus that the main value of MTC would be to exclude the diagnosis of asthma21; however, its results should be interpreted considering the clinical asthma context (treatment, environmental pollution, population characteristics) and thinking that other conditions, and even normal children, could have positive MTC.16 In asthmatic children, BHR to methacholine decreases during treatment with ICS, coinciding with symptoms improvement;20,22–24 furthermore, it has been found that BHR at 10 years of age predicts active asthma six years later.28 Overall, MTC is consistently positive in asthmatic children, regardless of atopy and ICS treatment, and despite differences in methodology and definitions of BHR, and this transversally reported finding would support its diagnostic value for asthma under well-defined clinical and methodological conditions.8–11,16,18,21,23–28 At present there is no consensus on what the cut-off value is when using methacholine challenge test to define BHR in asthmatic children. In patients aged seven years, it was found that using a cut-off value of PC20 3 mg/mL resulted in the maximal sum of sensitivity plus specificity,26 other authors have used a cut-off value of 16 mg/ml.25 A recent technical standard on bronchial challenge testing21 categorised the airway response to methacholine as normal when PC20 was higher than 16 mg/mL; airway hyperresponsiveness (AHR) was classified as borderline (PC20 4−16 mg/mL), mild (PC20 1−4 mg/mL), moderate (PC20 0.25−1 mg/mL) and marked (PC20 < 0.25 mg/mL).
Although increased FENO would mainly reflect eosinophilic airway inflammation,12,29 we found that abnormally elevated FENO was present in less than half of our atopic asthmatic children, which may suggest that an important proportion of them would not have eosinophilic airway inflammation at the time of measurement. The lack of airway inflammation in asthmatic adolescents assessed using sputum analysis has been recently reported.30 Another possible explanation may be the effect of ICS on decreasing FENO, as previously found in asthmatic children.10,11 Overall, our study indicates that FENO by itself has a limited role for corroborating the diagnosis of childhood asthma which agrees with a recent metanalysis reporting a modest performance of FENO for diagnosing asthma in children.31 If we had used a FENO cut off >35 ppb,12 only 25% of our patients could have been corroborated as asthmatics.
Probably the most widespread test for asthma diagnosis in children is the demonstration of BDR to short-acting beta two agonists, mainly because when positive it reflects, together with BHR, two fundamental features of asthma; however, there is no global consensus on the definition of bronchial reversibility in children. The usually employed cut-off of ≥12% FEV1 to define BDR is mainly derived from adult studies, and its diagnostic value for establishing asthma in children has been questioned because the sensitivity changes occur when using different cut-off values.4,15,25,26,32–34 In asthmatic adults a FEV1 ≥ 10% cut-off would be a good index for detecting non-controlled asthma in the long term35 but as yet there is no information on this respect in asthmatic children. In addition, it has been suggested that a positive BDR in patients on regular asthma treatment indicates poor asthma control.36 Almost all of our patients had normal baseline spirometry, which is a common finding in asthmatic children both at clinical and research settings;4–7,15,21,24,25,32,33,37 this situation raises an important challenge to clinicians on what tests should follow for establishing or corroborating the diagnosis of asthma in those patients.34 Our finding that only a low percentage of the asthmatic children with normal spirometry had positive BDR agrees with a recent study which found that only 4.9% of asthmatic children with normal spirometry had a BDR (ΔFEV1 ≥ 12%).38 This is a central issue since the diagnosis of asthma at primary and secondary care is still thought to be made in patients with abnormal spirometry or positive BDR. This could partly explain the low rate of asthma diagnosis found in referred children and adolescents who have suffered from “recurrent wheezing” for years, with many of them on long-term treatment with ICS. The present evidence suggests that waiting for an abnormal spirometry and positive bronchial reversibility to make the diagnosis of asthma in children appears as an unrealistic diagnostic approach.
There is not a single best method for studying asthmatic children with normal spirometry, and none of the common asthma tests used in children for detecting airway inflammation, BHR or changes in lung function after bronchodilators can establish the diagnosis of asthma by itself.4–6,9,12,16,23,27,30–34,37–39 Perhaps when there is concurrent normal spirometry, normal BDR and normal FENO, then MTC or other tests for detecting BHR could be a useful contribution for asthma diagnosis and management in these children,17,23,26,27 particularly if they are on regular ICS. On the other hand, a normal spirometry with a significant BDR at any time during follow-up, even in patients reported as asymptomatic, will most likely suggest a reactivation of asthma or a probable failure of treatment secondary to poor adherence to prescription and inhalation technique, or insufficient controller dose, among others.
Some authors have reported that using FEF25-75% instead of FEV1, may offer an advantage to detect a functional defect of the asthmatic airway because it correlates with BDR and MTC, and it would be a more sensitive indicator of symptomatic asthma than FEV1 in children, mainly in those with normal spirometry.37 Accordingly, in our study FEF25-75% showed better correlation with MCT, FENO and BDR than FEV1, and double the number of our patients would have been diagnosed as asthmatics if using a FEF25-75% cut-off value of 30% as positive BDR, instead of FEV1 ≥ 12%. It has been found that positive BDR defined as a FEF25−75 ≥ 30% change after bronchodilator identified a higher number of asthmatic children as compared to FEV1.19 However, evidence to define and standardise the actual role of FEF25-75% in the diagnosis and management of childhood asthma is still pending.
This study has several limitations, most of them inherent to having been performed in a real-life clinical situation, which could be commonly found at the daily practice when receiving referred patients as those in the present study. Thus, its cross-sectional design, selected sample (just those referred), all the patients on ICS treatment, consecutive patients’ assignment to tests, among others, would limit interpretations. In addition, the impossibility of verifying previous adherence to treatment and inhalation technique are among other difficult-to-control variables; however, these are real-life clinical conditions in which many of these children are usually referred to specialised centres and although they are seldom reported, occur frequently in daily clinical practice. An advantage of this study would be that all asthma tests were made within a few days of the respiratory physicians establishing the clinical diagnosis of asthma, before medication adjustments could alter the result of the tests. It has been shown that a significant improvement or even normalisation of some asthma tests occurs within one and three months after starting ICS treatment.10,24,28 Another strength would be the demonstration of the limitation that a diagnostic approach mainly based on the positive results of common asthma tests would have, particularly under daily clinical practice situations, such as those of this study.
The concern that only using clinical bases for asthma diagnosis would result in an overdiagnosis of asthma in children is unfounded because there is no solid evidence supporting that assumption. The reported low diagnostic value of tests or algorithms for diagnosing asthma indicates that at least at present, the diagnosis of asthma in children should still be based on clinical criteria, particularly considering that underdiagnosis has much more negative clinical and epidemiological implications for patients and public health.1 Our study contributes to the present knowledge on the low efficacy of current asthma tests for establishing or corroborating asthma diagnosis in children; however, when abnormal, they are a strong contribution for the diagnosis and treatment strategies of childhood asthma. Repetition of conventional asthma tests during follow-up could be useful for assessing the effect of treatment and to define variation of patient’s physiological and inflammatory measurements during symptomatic and asymptomatic periods; this seems to be a stronger indication for those tests in the modern management of asthma in children.
The insufficiency of asthma tests and asthma algorithms to corroborate the diagnosis in children with current asthma has recently been seen in a cohort study5 where authors found that when using a guideline-suggested2 algorithm for asthma diagnosis “only 2.2% of symptomatic children met the algorithm definition of asthma, but neither met the epidemiological definition”, indicating about the weakness and scarcity of current evidence on the clinical usefulness of common asthma tests for diagnosing current asthma in children.37–39 We think these limitations should be considered and highlighted in guidelines recommending clinicians to use asthma tests, either to establish or corroborate the diagnosis of asthma in children.
The findings of the present study may be useful for those physicians working at different level of healthcare who must daily confirm, or discard, the diagnosis of asthma in children, particularly those on ICS. At present, there are important doubts on the role and usefulness of conventional asthma tests for corroborating asthma in children at the daily clinical practice, and no test seems to offer a clear advantage over the clinical diagnostic criterion for that purpose.
ConclusionsOur study, performed under real-life clinical situations, showed that spirometry, BDR and FENO contribute little in corroborating the diagnosis of asthma in children on treatment with ICS. Asthma diagnosis made by respiratory physician strongly predicted BHR to methacholine, suggesting that MCT would have a role to confirm asthma diagnosis in children on treatment with ICS, but this needs further demonstration. Until there is more evidence from studies specifically designed to determine the role of asthma tests in children, performed under daily clinical situations, the asthma diagnosis corroboration in paediatric patients on ICS treatment should be based on the classical medical criteria, independently of using functional and inflammatory measurements for assessing the management and progression of the condition.
Ethical disclosuresProtection of human subjects and animals in research. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that no patient data appear in this manuscript.
Right to privacy and informed consent. The authors declare that no patient data appear in this manuscript.
Author ContributionsAll authors participated in conception and design; data collection, analysis, and interpretation; drafting and revising manuscript, and approval of final manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors thank all the children who participated in the study and their parents. We thank Elba Soto and Isabel Bacigalupo for their valuable paramedical collaboration in this study.