Surgical site infection (SSI) in vascular surgery is a complication that may lead not only to healing problems, but also limb loss and risk of death. The incidence of surgical site infection at the groin after vascular procedures ranges among 3% to 44%. The aim of this study is to review the incidence of groin infection in our department and the degree of correlation between infection, known risk factors and preventing measures.
MethodsRetrospective review of consecutive arterial vascular surgeries in a university central hospital during one year. Patients undergoing groin incisions were studied according to baseline demographics, clinical characteristics, active infection, previous inguinal access, antithrombotic therapy, indication for intervention, prophylactic antibiotic use, type of intervention and type of graft used. Wound site infections over a 30-day period were registered and graded based on Szilagyi classification. On patients with documented groin infection, the presentation timing, modality of treatment (surgical and non-surgical) and time of healing were recorded. Data were analyzed by using SPSS 18.0 version software.
ResultsFrom January to December 2013, 1266 vascular surgeries were performed. Of these, 782 were arterial vascular procedures, 279 by inguinal approach, with a total of 358 groin incisions. Our base population included 241 patients (31 patients with more than one procedure in a year). Total infection rate was 4.7% (17/358 groin incisions). All Szilagyi I (n=4) resolved with antibiotic only; three of the ten Szilagyi II infections needed drainage/debridement at operation room; the other Szilagyi II resolved with antibiotic and wound care. All Szilagyi III infections (n=3) were treated with bypass removal, and in two patients an in situ revascularization was performed. These two patients died from sepsis. Identification of the infecting agent was possible in 9 patients, with the most common isolated agent being Pseudomonas. Hypertension was significantly associated with a higher risk of SSI (p=0.033).
Discussion/ConclusionsIn our department, the infection rate of the inguinal approach equals the best international standards described. These results may be the outcome of a carefully planned strategy of infection control. Arising from the fact that groin SSI is a low incidence event, correlations between suspected risk factors and this infection are hard to establish. Longer follow-ups would be useful for identification of all groin infections.
A infeção do local cirúrgico na cirurgia vascular é uma complicação que não só implica atrasos na cicatrização, mas que pode levar a perda de membro e a risco de vida para o paciente. A incidência descrita de infeção da ferida cirúrgica inguinal após procedimentos vasculares varia de 3 a 44%. Este estudo tem como objetivo quantificar a incidência de infeção inguinal no nosso serviço e estabelecer níveis de correlação com fatores de risco e medidas preventivas.
MétodosEstudo retrospetivo das cirurgias vasculares arteriais realizadas num hospital universitário durante um ano. Pacientes com abordagem inguinal foram estudados segundo características clínicas e demográficas, infeção ativa, acesso inguinal prévio, terapia antitrombótica, indicação cirúrgica, antibioterapia profilática, tipo de intervenção e tipo de enxerto. Foram registadas as infeções do local cirúrgico e classificadas segundo Szilagyi. Nos pacientes com infeção, documentamos o tempo de apresentação, tratamento e tempo de cicatrização. Os dados foram analisados no SPSS 18.0.
ResultadosDe janeiro a dezembro de 2013, foram realizadas 1266 cirurgias vasculares, das quais 782 arteriais. Destas, 279 tiveram abordagem inguinal, com um total de 358 incisões inguinais e uma amostra populacional de 241 pacientes (31 reintervencionados). A taxa de infeção foi de 4,7% (17/348 incisões). Todas as infeções Szilagyi I (n=4) resolveram com uso exclusivo de antibiótico. Três das dez infeções Szilagyi II necessitaram de drenagem/desbridamento no bloco; as restantes Szilagy II resolveram com antibiótico e cuidados de penso. Nas infeções Szilagyi III (n=3) foi retirada a pontagem, e em dois doentes foi tentada a revascularização in situ. Esses dois pacientes morreram. A identificação do agente infecioso foi possível em 9 pacientes, sendo a Pseudomonas o agente mais comum. A hipertensão esteve significativamente associada a maior risco de infeção (p=0,033).
Discussão/ConclusõesA baixa taxa de infeção descrita é comparável aos melhores resultados publicados sobre este tema. Estratégias de controlo de infeção refinadas ao longo de anos podem contribuir para estes resultados. Contudo, sendo um evento de baixa incidência, correlações entre infeção da ferida inguinal e fatores de risco são difíceis de estabelecer. Períodos de seguimento mais longos são necessários para a identificação de todas infeções a este nível.
Surgical site infection (SSI) in vascular surgery is a complication that may lead not only to healing problems, but also limb loss and risk of death. It always implies increased hospital stay, hospital costs and morbidity for the patient.1
Groin incisions play a central role in various vascular surgical procedures. In addition to their traditional use in bypass procedures, endarterectomies and thromboembolectomies, more than 95% of all percutaneous interventions are performed by femoral access.2
The incidence of surgical site infection at the groin after vascular procedures ranges from 3% to 44%.3 Factors contributing to the increased incidence of SSI in this subset of patients include disruption of lymphatics, proximity to the perineum and prosthetic graft placement. The increasing prevalence infection by multiresistant bacteria has been associated with worsened outcomes.4
Although the management of groin SSI is well documented, there is little published about the incidence and classification of these infections. Szilagyi et al said that diabetes, as well as obesity, had a relatively small role in initiating groin wound infection.5 A recent study showed that redo bypass, female gender, diabetes and active infection were associated with a risk of graft infection involving the femoral artery.6
Besides the general aseptic measures, the only strategy that significantly reduces the incidence of SSI is prophylactic systemic antibiotics administered only preoperatively.4
There is no “gold standard” for treatment of these infections. The initial treatment generally includes surgical debridement and antibiotics. Secondary interventions have been attempted with limited success, including rotational flaps, wound vacuum-assisted closure, alginate dressing, graft excision and extra-anatomic or in situ bypass or patch replacement using autologous, rifampin-soaked or silver-impregnated materials.7–10The aim of this study is to describe the incidence of groin infection in our vascular department and the degree of correlation between infection/known risk factors and infection/preventing measures.
MethodsRetrospective review of consecutive arterial vascular surgeries in a central operating room during one year. Patients undergoing groin incisions for arterial vascular procedures were studied according to baseline demographics, clinical characteristics, active infection (ipsilateral lower extremity infection occurring before vascular surgery), previous inguinal access (including arterial puncture), antithrombotic therapeutic in use, initial indication for intervention, type of intervention and type of graft used.
We reported the universal good care practice that every patient was subjected to before going to the operating room.
We defined patients under prophylactic antibiotic those who received intraoperative antibiotic or patients who were under antibiotic therapy at the time of surgery.
All patients underwent longitudinal incision for femoral cutdown.
Past medical history of diabetes mellitus, hypertension, cerebrovascular disease, coronary artery disease, smoking – including both current and former smokers (>6 months), end-stage renal disease on hemodyalisis and history of kidney transplant, were recorded.
The diagnostic criteria for SSI included signs/symptoms of infection (pain, tenderness, erythema and/or swelling), purulent drainage from the wound and organisms isolated from aseptically smears cultures of fluid or tissue.
Wound site infections over a 30-day period were registered and graded based on Szilagyi classification.5 Grade I infections had only dermal involvement. Grade II infections extended to the subcutaneous region and included dehiscence of wound. Infections involving arterial graft were grade III. Patients with hematomas and noninfected lymphoceles were also accounted.
On patients with documented groin infection it was registered the presentation timing, modality of treatment (surgical and non-surgical) and time of healing.
Data were entered in Microsoft Excel 2010 and analyzed by using SPSS 18.0 version software.
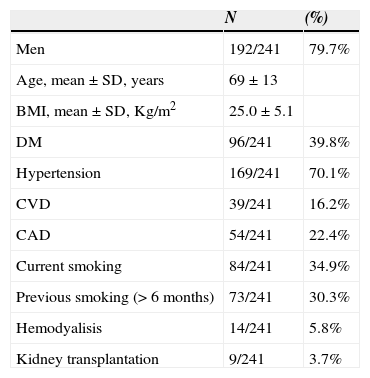
ResultsFrom January to December 2013, 1266 vascular surgeries were performed at our central operating rooms. Of these, 782 were arterial vascular procedures, 279 by inguinal approach, with a total of 358 groin incisions. Our base population had 241 patients (31 patients with more than one procedure in a year). Demographics and clinical characteristics of our population are presented in Table 1.
Patient Demographic and Clinical Characteristics.
| N | (%) | |
|---|---|---|
| Men | 192/241 | 79.7% |
| Age, mean±SD, years | 69±13 | |
| BMI, mean±SD, Kg/m2 | 25.0±5.1 | |
| DM | 96/241 | 39.8% |
| Hypertension | 169/241 | 70.1% |
| CVD | 39/241 | 16.2% |
| CAD | 54/241 | 22.4% |
| Current smoking | 84/241 | 34.9% |
| Previous smoking (> 6 months) | 73/241 | 30.3% |
| Hemodyalisis | 14/241 | 5.8% |
| Kidney transplantation | 9/241 | 3.7% |
* SD, Standard Deviation; BMI, Body Mass Index; DM, Diabetes Mellitus; CVD, Cerebrovascular Disease; CAD, Coronary Artery Disease.
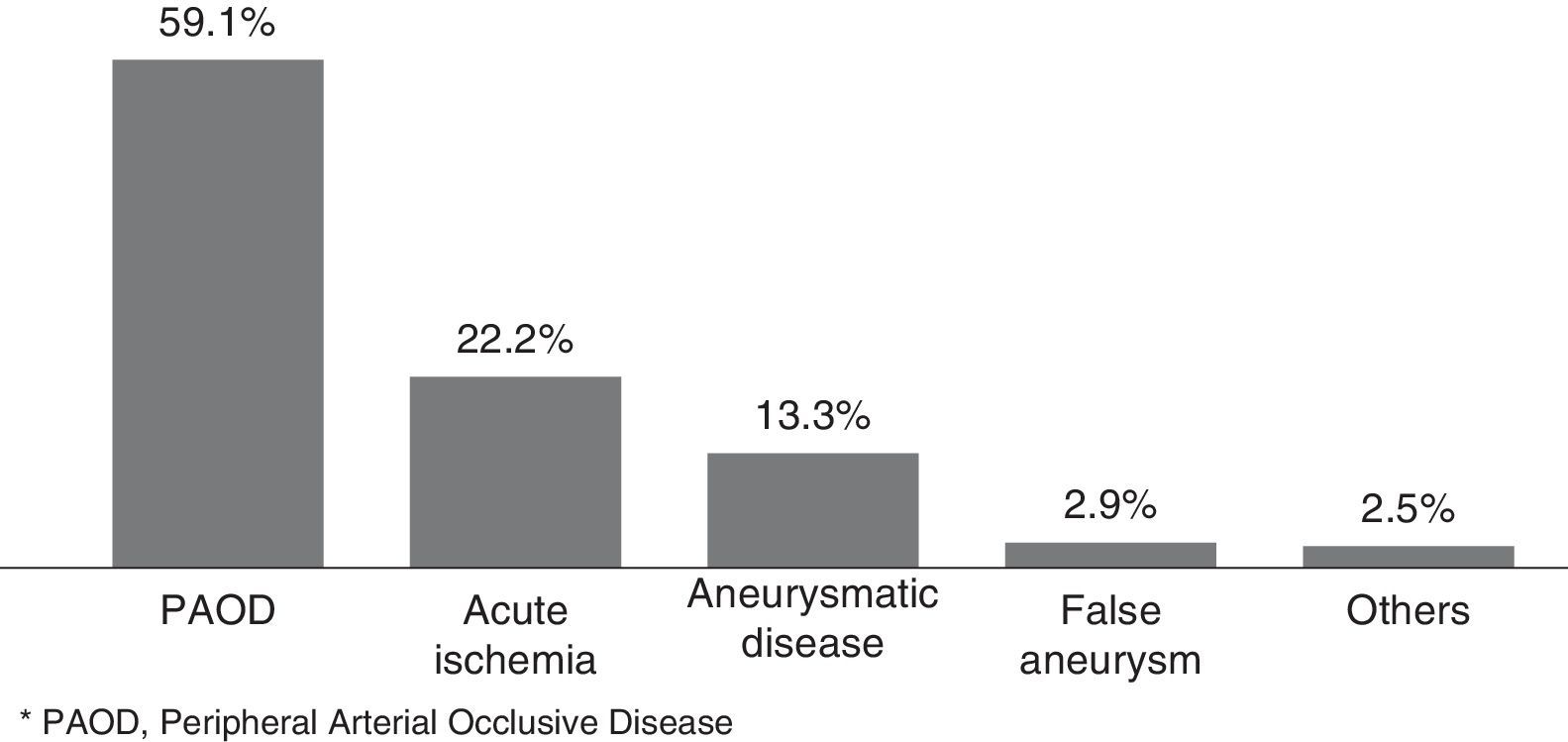
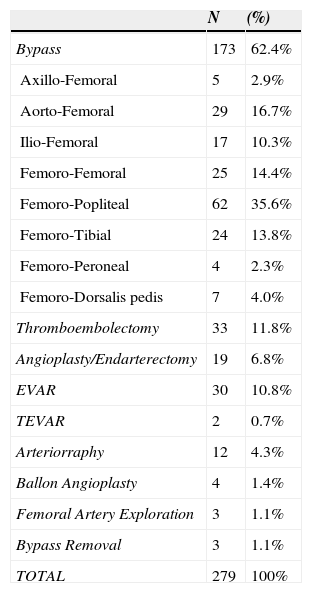
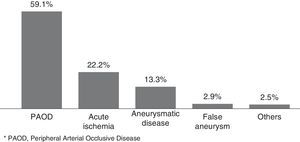
In Figure 1, we illustrated the indications for the 279 interventions, and in Table 2 we listed the procedures performed. A graft prosthesis was used in 114 of the 173 bypass surgeries (65.9%). Once the patient was considered infected, no more procedures were included in our series. The perioperative characteristics are described in Table 3.
Procedures.
| N | (%) | |
|---|---|---|
| Bypass | 173 | 62.4% |
| Axillo-Femoral | 5 | 2.9% |
| Aorto-Femoral | 29 | 16.7% |
| Ilio-Femoral | 17 | 10.3% |
| Femoro-Femoral | 25 | 14.4% |
| Femoro-Popliteal | 62 | 35.6% |
| Femoro-Tibial | 24 | 13.8% |
| Femoro-Peroneal | 4 | 2.3% |
| Femoro-Dorsalis pedis | 7 | 4.0% |
| Thromboembolectomy | 33 | 11.8% |
| Angioplasty/Endarterectomy | 19 | 6.8% |
| EVAR | 30 | 10.8% |
| TEVAR | 2 | 0.7% |
| Arteriorraphy | 12 | 4.3% |
| Ballon Angioplasty | 4 | 1.4% |
| Femoral Artery Exploration | 3 | 1.1% |
| Bypass Removal | 3 | 1.1% |
| TOTAL | 279 | 100% |
EVAR, Endovascular Aneurysm Repair; TEVAR, Thoracic Endovascular Aortic Repair.
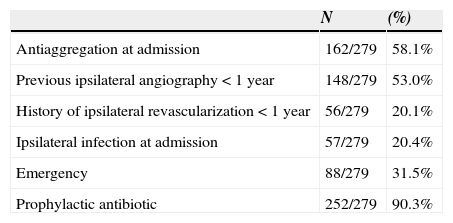
Perioperative characteristics.
| N | (%) | |
|---|---|---|
| Antiaggregation at admission | 162/279 | 58.1% |
| Previous ipsilateral angiography < 1 year | 148/279 | 53.0% |
| History of ipsilateral revascularization < 1 year | 56/279 | 20.1% |
| Ipsilateral infection at admission | 57/279 | 20.4% |
| Emergency | 88/279 | 31.5% |
| Prophylactic antibiotic | 252/279 | 90.3% |
All patients received the universal good care practice before surgery: previous bath with clorohexidine, trichotomy immediately before surgery, skin antisepsis with alcoholic solution, intraoperative glove change, closed suction drain placement, careful haemostasis and layered closure. Prophylactic antibiotic was administered to 90.3% of the patients.
Total infection rate was 4.7% (17/358 groins incisions), representing complication in 16 out of the 279 procedures (5.7%). One patient had bilateral groin involvement. There were 4 patients (4 groins) who had Szilagyi I infection, 9 patients (10 groins) with Szilagyi II and 3 patients (3 groins) with Szilagyi III infection.
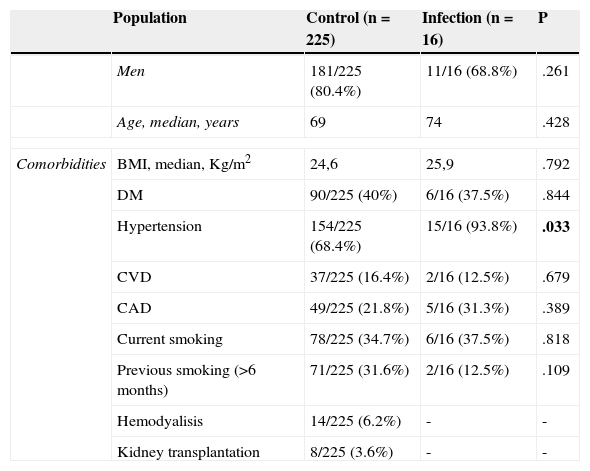
The demographics, clinical and preoperative characteristics of both infection and control group were compared and listed in Table 4. Hypertension was significantly associated with a higher risk of SSI (p=0.033).
Comparison of patient demographics, clinical and preoperative characteristics between Control group and Infection group.
| Population | Control (n=225) | Infection (n=16) | P | |
|---|---|---|---|---|
| Men | 181/225 (80.4%) | 11/16 (68.8%) | .261 | |
| Age, median, years | 69 | 74 | .428 | |
| Comorbidities | BMI, median, Kg/m2 | 24,6 | 25,9 | .792 |
| DM | 90/225 (40%) | 6/16 (37.5%) | .844 | |
| Hypertension | 154/225 (68.4%) | 15/16 (93.8%) | .033 | |
| CVD | 37/225 (16.4%) | 2/16 (12.5%) | .679 | |
| CAD | 49/225 (21.8%) | 5/16 (31.3%) | .389 | |
| Current smoking | 78/225 (34.7%) | 6/16 (37.5%) | .818 | |
| Previous smoking (>6 months) | 71/225 (31.6%) | 2/16 (12.5%) | .109 | |
| Hemodyalisis | 14/225 (6.2%) | - | - | |
| Kidney transplantation | 8/225 (3.6%) | - | - | |
| Procedures | Control (n=263) | Infection (n=16) | P | |
|---|---|---|---|---|
| Perioperative Characteristics | Antiaggregation at admission | 152/263 (58%) | 10/16 (62,5%) | .724 |
| Previous ipsilateralangiography < 1 year | 140/263 (53.2%) | 9/16 (56.3%) | .791 | |
| History of ipsilateral revascularization < 1 year | 53/263 (19.8%) | 3/16 (18.8%) | .857 | |
| Ipsilateral infection at admission | 55/263 (20.9%) | 2/16 (12.5%) | .418 | |
| Emergency | 81/263 (30.8%) | 7/16 (43.8%) | .279 | |
| Prophylactic antibiotic | 239/263 (90.8%) | 13/16 (81.3%) | .258 | |
* BMI, Body Mass Index; DM, Diabetes Mellitus; CVD, Cerebrovascular Disease; CAD, Coronary Artery Disease.
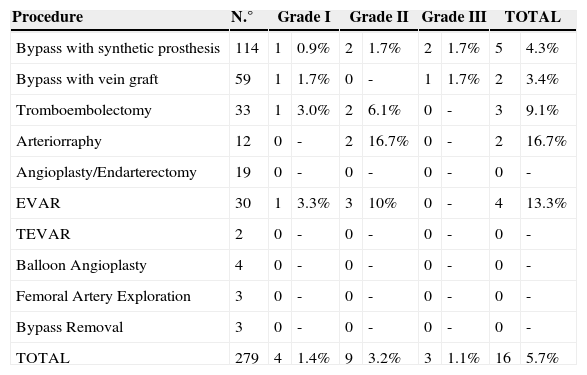
The time to presentation with infection ranged from 2 to 25 days (mean, 13±8). Incidence of infection by type of procedure was described in Table 5.
Incidence of infection by type of procedure.
| Procedure | N.° | Grade I | Grade II | Grade III | TOTAL | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bypass with synthetic prosthesis | 114 | 1 | 0.9% | 2 | 1.7% | 2 | 1.7% | 5 | 4.3% |
| Bypass with vein graft | 59 | 1 | 1.7% | 0 | - | 1 | 1.7% | 2 | 3.4% |
| Tromboembolectomy | 33 | 1 | 3.0% | 2 | 6.1% | 0 | - | 3 | 9.1% |
| Arteriorraphy | 12 | 0 | - | 2 | 16.7% | 0 | - | 2 | 16.7% |
| Angioplasty/Endarterectomy | 19 | 0 | - | 0 | - | 0 | - | 0 | - |
| EVAR | 30 | 1 | 3.3% | 3 | 10% | 0 | - | 4 | 13.3% |
| TEVAR | 2 | 0 | - | 0 | - | 0 | - | 0 | - |
| Balloon Angioplasty | 4 | 0 | - | 0 | - | 0 | - | 0 | - |
| Femoral Artery Exploration | 3 | 0 | - | 0 | - | 0 | - | 0 | - |
| Bypass Removal | 3 | 0 | - | 0 | - | 0 | - | 0 | - |
| TOTAL | 279 | 4 | 1.4% | 9 | 3.2% | 3 | 1.1% | 16 | 5.7% |
* EVAR, Endovascular Aneurysm Repair; TEVAR, Thoracic Endovascular Aortic Repair.
Previous revascularization with open surgery involved the ipsilateral inguinal area in 18.8% of the infected patients (3/16) and 56.3% had a history of previous femoral artery puncture (9/16).
Identification of the infecting agent was possible only in 9/16 patients. The most common isolated agent was Pseudomonas aeruginosa (3/16).
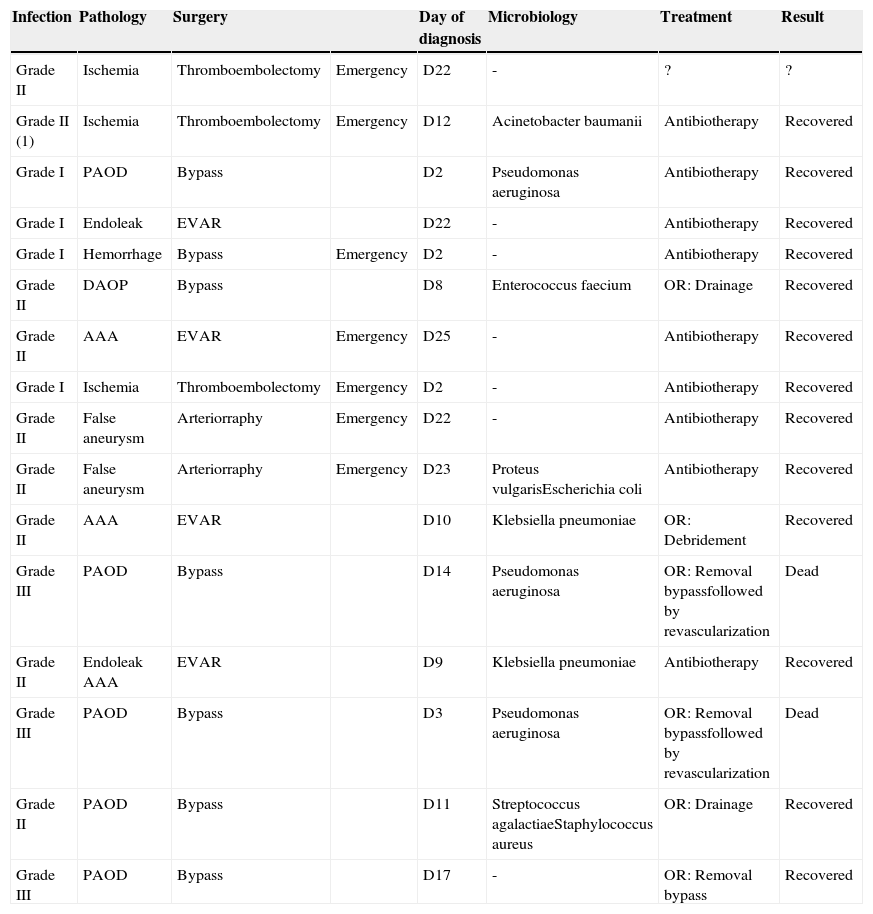
All infections were described in Table 6. Empiric antibiotic treatment was instituted after clinical diagnosis of wound infection. All Szilagyi I resolved with antibiotic only; Three patients with Szilagyi II infections needed drainage/debridement on operation room – the others Szilagyi II resolved with antibiotic and wound care. All Szilagyi III were treated with bypass removal, and in two patients, an in situ revascularization was performed. These two patients died from sepsis.
Detailed description of all infections.
| Infection | Pathology | Surgery | Day of diagnosis | Microbiology | Treatment | Result | |
|---|---|---|---|---|---|---|---|
| Grade II | Ischemia | Thromboembolectomy | Emergency | D22 | - | ? | ? |
| Grade II (1) | Ischemia | Thromboembolectomy | Emergency | D12 | Acinetobacter baumanii | Antibiotherapy | Recovered |
| Grade I | PAOD | Bypass | D2 | Pseudomonas aeruginosa | Antibiotherapy | Recovered | |
| Grade I | Endoleak | EVAR | D22 | - | Antibiotherapy | Recovered | |
| Grade I | Hemorrhage | Bypass | Emergency | D2 | - | Antibiotherapy | Recovered |
| Grade II | DAOP | Bypass | D8 | Enterococcus faecium | OR: Drainage | Recovered | |
| Grade II | AAA | EVAR | Emergency | D25 | - | Antibiotherapy | Recovered |
| Grade I | Ischemia | Thromboembolectomy | Emergency | D2 | - | Antibiotherapy | Recovered |
| Grade II | False aneurysm | Arteriorraphy | Emergency | D22 | - | Antibiotherapy | Recovered |
| Grade II | False aneurysm | Arteriorraphy | Emergency | D23 | Proteus vulgarisEscherichia coli | Antibiotherapy | Recovered |
| Grade II | AAA | EVAR | D10 | Klebsiella pneumoniae | OR: Debridement | Recovered | |
| Grade III | PAOD | Bypass | D14 | Pseudomonas aeruginosa | OR: Removal bypassfollowed by revascularization | Dead | |
| Grade II | Endoleak AAA | EVAR | D9 | Klebsiella pneumoniae | Antibiotherapy | Recovered | |
| Grade III | PAOD | Bypass | D3 | Pseudomonas aeruginosa | OR: Removal bypassfollowed by revascularization | Dead | |
| Grade II | PAOD | Bypass | D11 | Streptococcus agalactiaeStaphylococcus aureus | OR: Drainage | Recovered | |
| Grade III | PAOD | Bypass | D17 | - | OR: Removal bypass | Recovered |
* PAOD, Peripheral Arterial Occlusive Disease; AAA, Abdominal Aortic Aneurysm; EVAR, Endovascular Aneurysm Repair; OR, Operation Room.
(1) Bilateral infection.
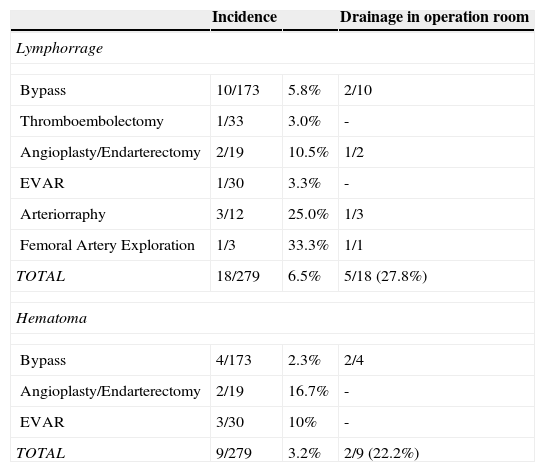
Other wound complications were registered and mentioned in Table 7.
Other complications per procedure.
| Incidence | Drainage in operation room | ||
|---|---|---|---|
| Lymphorrage | |||
| Bypass | 10/173 | 5.8% | 2/10 |
| Thromboembolectomy | 1/33 | 3.0% | - |
| Angioplasty/Endarterectomy | 2/19 | 10.5% | 1/2 |
| EVAR | 1/30 | 3.3% | - |
| Arteriorraphy | 3/12 | 25.0% | 1/3 |
| Femoral Artery Exploration | 1/3 | 33.3% | 1/1 |
| TOTAL | 18/279 | 6.5% | 5/18 (27.8%) |
| Hematoma | |||
| Bypass | 4/173 | 2.3% | 2/4 |
| Angioplasty/Endarterectomy | 2/19 | 16.7% | - |
| EVAR | 3/30 | 10% | - |
| TOTAL | 9/279 | 3.2% | 2/9 (22.2%) |
* EVAR, Endovascular Aneurysm Repair.
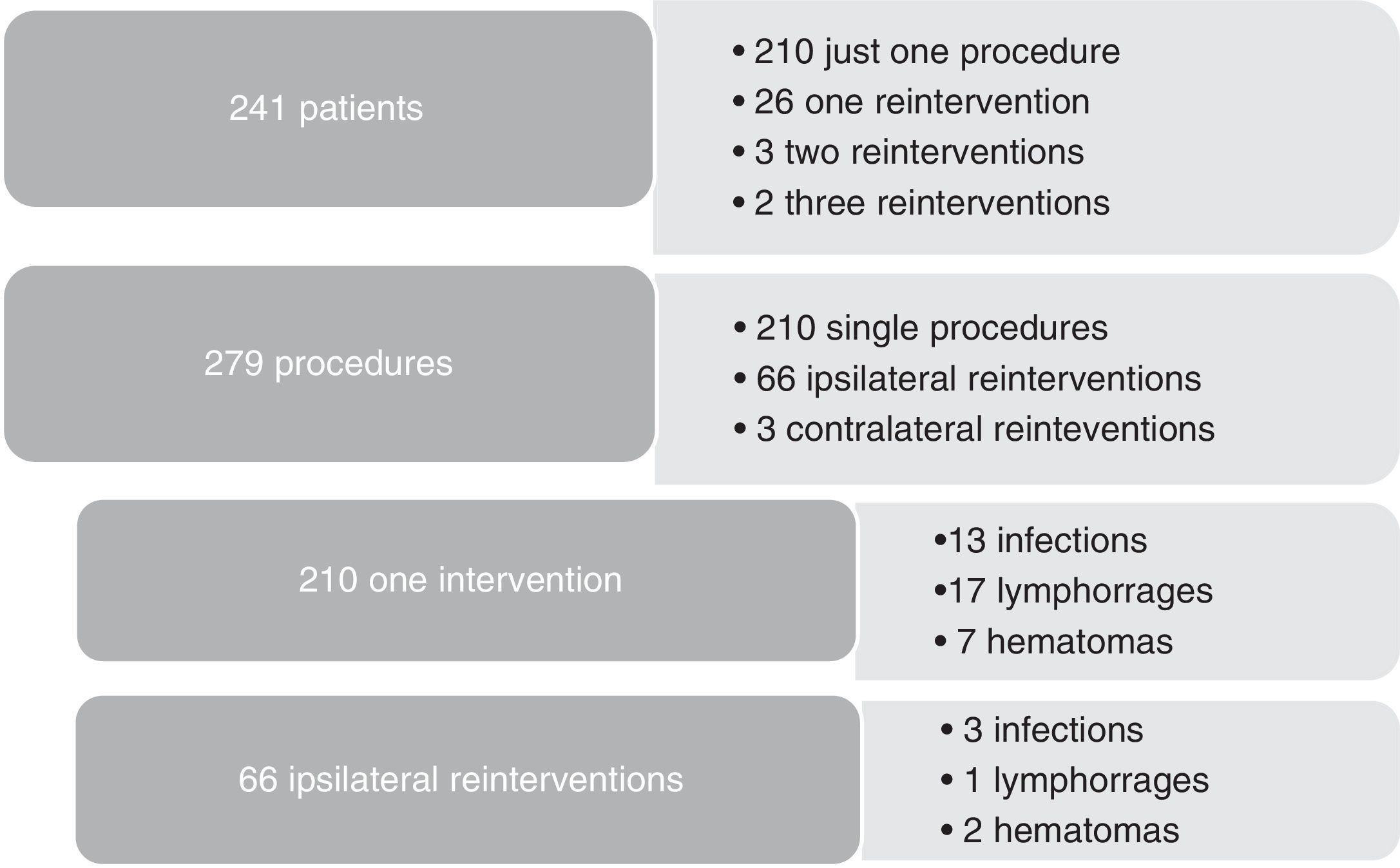
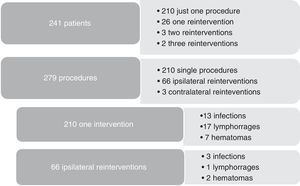
As redo operations are a consistent risk factor for surgical site infections, we compared the infection rates according to the number of reinterventions (Figure 2). Three out of 31 reoperated patients were subjected to contralateral procedures, so they were not truly redo operations and were excluded, leaving 28 redo patients to compare with 210 patients treated with just one procedure during 2013. Subgroup analysis showed that hemodialysis was a risk factor for reintervention (5/28 vs 8/210, p=0.002). As for complications after reintervention, we had 3/66 infections, 1/66 lymphorrage and 2/66 hematomas. No statistically significant difference was found in the outcomes between re-operated patients and one-time operated patients.
DiscussionMost wound complications (hematoma, seroma and dehiscence) and superficial infections of surgical site are easily treated, but frequently neglected by the vascular surgeon. However, they are assumed risk factors for deeper infections extending to the graft, which are associated with catastrophic consequences: death rate between 15 to 75% and major amputations rate up to 70%.11 Even when the treatment is successful, the morbidity associated with vascular graft infection is considerable, the outcomes often being worse than the natural history of the vascular condition that led to graft implantation.2 Vascular surgeons should maintain a low threshold for proceeding with additional diagnostic testing when any symptom or sign suggests graft infection.
In a case-controlled study reported by Antonios et al., multivariate analysis identified only groin incision and wound infection as factors increasing the risk of subsequent vascular graft infection.12 Dennis F Bandyk added that, for the vascular patient, obesity, advanced age, smoking and diabetes were characteristics that potentiate SSI.1 In our study we found that hypertension rate was higher in patients that got infection (p=0.03), but no other study found this correlation.
We had an incidence of SSI at the groin after vascular procedures of 4.7%: a good mark comparing with an overall incidence described in the range of 3 to 44%.3 However, a possible explanation for the differences in the reported incidence could be the definition of SSI used.
Most bacterial infections occur from direct spread of bacteria from the wound surface.4 Therefore, our focus must be in the prevention of surgical site infection. In our center antibiotic prophylaxis was administered to 90.3% of the patients. There is clear evidence that this is effective for SSI prevention in vascular surgery. Other protocol prophylactic measures used (previous bath with chlorhexidine, trichotomy immediatly before surgery, skin antisepsis with alcoholic solution, intraoperative glove change, closed suction drain placement, careful hemostasis and layered closure) are recommended but do not have the same level of evidence.4
The prophylactic antibiotic of choice in our department is cefazolin.13,14 Cefazolin is a first-generation, low cost, low toxicity cephalosporin, active against methicillin-sensitive Staphylococcus aureus, coagulase negative Staphylococcus and some Gram-negative bacilli. The initial dose is twice the usual (usually 1-2g EV) and, in longer surgeries, booster doses are administered every 4hours. Cefazolin is administered 60minutes before surgery and administration is completed before incision. In patients already under antimicrobial treatment an extra dose 60minutes before the surgical incision is administered.
We signalized a history of arterial puncture at the site of infection in 56.3% of the patients that develop SSI. The significant association described elsewhere between preoperative inguinal arteriography and groin infection confirms the necessity of adopting specific preventive measures to avoid the high morbidity and mortality inherent to this vascular surgery complication.15
Recently, negative pressure dressing combined with standard perioperative infection prevention has shown to significantly decrease the incidence of groin SSIs in patients undergoing vascular procedures in one single center.3
Although virtually any microorganism can infect a vascular prosthesis, S. aureus is the most prevalent pathogen and accounts for one fourth to one half of the infections depending on the implant site. Early infections are caused by virulent hospital-acquired bacteria and frequently associated with sepsis, as evidenced by fever, leukocytosis, bacteremia and an advanced wound infection. Late infections result from graft colonization by low-virulence organisms such as Staphylococcus epidermiditis. The low titer of microorganisms on graft surfaces produces an indolent infection without signs of sepsis, and cultures of perigraft fluid or tissue may yield no growth. Although typically less virulent, these infections are more established and difficult to eradicate locally.2 In our study, the highest incidence of infection with more aggressive pathogens was coincident with the characteristics of early infections and may support that prevention measures applied were effective in preventing wound infection by commensal bacteria of the skin flora.
The empirical antibiotic therapy should cover methiclin-resistant S. aureus and gram negative bacilli. Associations with beta lactams, daptomycin or vancomycin and aminoglycosides seems the best option.17 Among the anti-staphylococcal drugs available, the preference for daptomycin, rather than vancomycin, has been growing due to its bactericidal activity, ability to prevent bacterial adhesion, capacity to penetrate into biofilm and to prevent the formation of biofilm itself.16 A synergistic effect when used with rifampin has been described.17
Multiple surgical treatment modalities have been attempted with limited success and increased health care costs, including rotational flaps, wound vacuum-assisted closure and excision of prostheses with extra-anatomic bypass. In femorodistal bypass, in the absence of bleeding or severe sepsis, aggressive local treatment with debridement and drainage of localized collections, bactericidal broad-spectrum antibiotics and use of muscle flaps, was associated with lower rates of limb amputation and with reinfection and mortality rates similar to conventional therapy which involves the excision of the graft, with or without arterial reconstruction.6,18,19 In our study, the two patients with graft infection and in whom revascularization was tried, did not survive. These two fatal results are a red flag for the devastating consequences that may arise from surgical site infection, and may be a stop to endless revascularization attempts.
ConclusionsIn addition to showing our low incidence rate of groin SSI, this study offers the opportunity to review risk factors, preventive measures and therapeutic modalities in vascular infections. Our rate of groin infection is consistent with the best international reports. Strategies for infection control refined over years may contribute to these excellent results. The difficulty in establishing a correlation between infection/risk factors and infection/preventing measures results from the low incidence of groin infection and the necessity for longer follow-up periods
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.