Introduction: Raynaud's phenomenon (RP) is a well-defined clinical syndrome. Systemic sclerosis (SSc) is the most frequent associated disease to RP (96%). The aim of this study was to assess the differences between primary RP (PRP) and secondary RP (SRP) regarding macrovascular disease parameters, endothelial dysfunction and angiogenesis biomarkers.
Materials and methodsFlow-mediated dilatation (FMD), endothelin-1 (ET-1), asymmetric dimethylarginine (ADMA) vascular endothelial growth factor (VEGF), endoglin and endostatin were analyzed in a cohort study of 32 PRP patients and 77 SRP all with SSc. 38 of the SRP SSc-associated patients had severe digital ulcer (DU).
ResultsPatients with PRP had significantly longer history of RP compared to SRP SSc-sssociated patients (p=0.028).
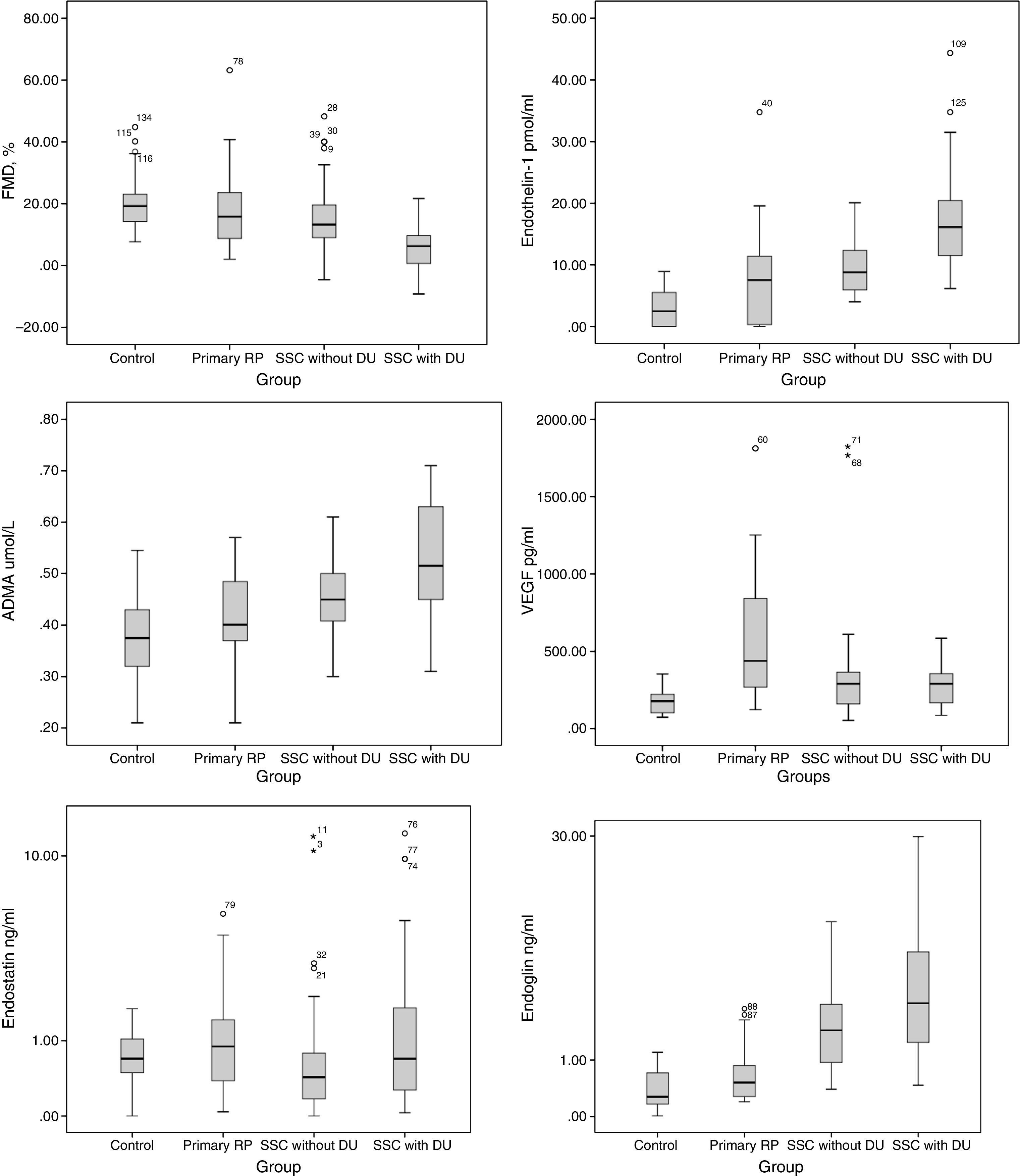
FMD was significantly lower in SRP patients 10.85±11.0% (p<0.001), more evidenced in SRP SSc-associated DU patients 5.34±7.49 (p<0.001). ET-1 plasma levels were significantly increased in both PRP 7.53 (0.16–11.73) and SRP patients 11.85 (7.42–17.23) (p<0.001). Significant increased serum levels of ADMA 0.52 (0.45–0.63)μmol/L (p<0.001) and endoglin 3.01 (1.46–7.02)mg/ml (p<0.001) were found in the SRP SSc-associated group with DU. VEGF was significantly decreased in the DU group 245.06 (158.68–347.33)pg/ml compared to PRP 438.50 (269.26-854.00)pg/ml and SRP naïve–DU patients 290 (166.71–361.78)pg/ml patients (p<0.001). No significant differences were found between groups regarding endostatin (p=0.118).
Comparing PRP and SRP SSc-associated patients without DU no statistically significant difference regarding FMD, ET-1, ADMA, VEGF, plasma levels were observed.
ConclusionOverproduction of ET-1 and VEGF is present in PRP patients. Macrovascular disease and an impaired response to shear stress are more characteristic of SRP with a grater expression in patients with peripheral ischemic lesions.
O Fenômeno de Raynaud (FR) é uma patologia clínica comum, caracterizada por episódios recorrentes de vasospasmo das artérias digitais desencadeados pela exposição ao frio ou pelo stress emocional. O FR pode ser classificado como sendo primário (FRP) idiopático ou secundário (FRS) se associado a outras patologias ou condições, a mais frequente a esclerodermia. O objectivo deste estudo foi a identificação de diferenças entre o FR primário e o secundário no que respeita a parâmetros de avaliação de doença macrovascular, disfunção endotelial e angiogenese.
Material e métodosForam analisados parâmetros clínicos e demográficos, avaliada a doença macrovascular com o teste de Allen e a fluxo mediada pelo dilatação (FMD), doença microvascular através da videocapilaroscopia periungueal (NVC), foi feita a pesquisa de autoanticorpos e medidos os biomarcadores de doença vascular de disfunção endotelial (a endotelina-1-ET-1 e dimetilarginina assimétrica- ADMA) e de angiogênese (fator de crescimento endotelial vascular- VEGF, endostatina e endoglina) em todos os doentes e no grupo controle.
ResultadosDoentes com FR primário tinham duração de doença superior aos FRS (p=0.028).
O FMD era significativamente menor nos doentes com FRS 10.85±11.0% (p<0.001), e nestes a resposta era pior nos doentes com úlcera activa 5.34±7.49 (p<0.001). Os níveis plasmáticos de ET-1 estavam significativamente aumentados nos doentes com FRP 7.53 (0.16-11.73) e nos FRS 11.85 (7.42-17.23) (p<0.001). No grupo com úlcera activa verificou-se níveis séricos aumentados de ADMA 0.52 (0.45-0.63)umol/L (p<0.001) e de endoglina 3.01 (1.46-7.02) mg/ml (p<0.001). Pelo contrário este grupo apresentava valores inferiores de VEGF 245.06 (158.68-347.33) pg/ml comparado aos FRP (269.26-854.00) pg/m e aos FRS sem úlcera 290 (166.71-361.78) pg/ml (p<0.001). No que respeita ao biomarcador angiostático endostatina não identificamos diferenças entre grupos (p=0.118).
Os doentes com FRP e os SRPN sem lesão vascular periférica não apresentavam diferenças significativas nos biomacadores estudados.
Conclusão: Os doentes com FRP tem níveis elevados de ET-1 e VEGF. A doença macrovascular com uma má resposta ao shear stress são características de doentes com FRS e lesão periférica isquémica.
Raynaud's Phenomenon (RP) was first described by Maurice Raynaud in 1862 and is defined as bouts of reversible vasospastic ischemia of the extremities.1,2 Episodic color changes of the fingers classically turn into white (ischemia), then blue (cyanosis) and red (reperfusion). In a recent Delphi exercise round, 12 invited experts agreed recently in three-step outline for a newly proposed diagnostic method. Consensus was achieved in that at least biphasic color changes are required to make the diagnosis of RP. They also agreed that white/pallor and blue/cyanosis were the two most important colors and that patients must report cold temperatures as one of the triggers for their RP attacks.3
Primary RP (PRP), also known as Raynaud's disease, is a functional vascular disorder that occurs isolated as an exaggerated response to cold and emotional stress, not progressing to irreversible tissue injury.3,4 The requests for definition of PRP defined in Delphi exercise round were: (i) normal capillaroscopy; (ii) negative physical examination for findings suggestive of secondary causes (e.g. ulcerations, tissue necrosis or gangrene, sclerodactily, calcinosis, or skin fibrosis); (iii) no history of existing connective tissue disease and (iv) negative or low titer ANA.3,5
Secondary RP (SRP), also known as Raynaud's Syndrome, appears in response to those triggers too, however, it occurs in the setting of underlying structural vascular disease and is often associated with digital ulceration, scarring or gangrene.6 Recent advances in the diagnosis of RP has have recognized that abnormalities in nailfold capillary pattern and specific autoantibodies are independent risk factors for connective tissue disease.4 Autoreactive antibodies specifically ANA, anti-centromere and anti-SCL 70 antibodies are helpful as diagnostic for secondary RP.
PRP is a common condition, which has a prevalence of 3–5% in the general population.2 The onset age is below 40 years and there could be a history of PRP in family but the entire clinical course is benign.6 By contrast, SRP is a much rarer condition, but frequent in the patients with connective diseases such as Systemic Sclerosis (SSc) (90%), systemic lupus erythematous (30%), rheumatoid arthritis (20%), Sjögren's syndrome and polymiositis.
Endothelial dysfunction and free-radical damage are primary events throughout the course of the RP disease, which result in vascular obliteration and diminished blood flow to the organs involved7 and are prominent features of RP and ischemic peripheral digital ulcers. There are several serological biomarkers that reflect the vasculopathy of the disease, such as vasoconstrictor ET-1,8 the controversial vasodilator nitric oxide(NO)8 and the inhibitor of endothelial NO synthase (eNOS) ADMA.
Endothelial cell damage results in ischemia-reperfusion injury due to the ongoing pathological process, which inevitably evolves toward chronic underperfusion. A characteristic clinical finding is capillary dilation and atrophy diagnosed by nailfold capillary microscopy. These findings suggest significant loss of the peripheral vascular network with a defect in both the vascular repair and in the expected increase in vessel growth (angiogenesis, arteriogenesis, vasculogenesis); the net result is tissue ischemia, fibrosis, and organ failure.9
The aim of the current study was to evaluate macrovascular disease parameters, endothelial dysfunction and angiogenic vascular biomarkers in a cohort of RP patients, in an attempt to define the boundaries between PRP and SRP allowing early identification of PRP patients who are at risk of developing an underling secondary disease.
Materials and methodsAn observational cohort study was conducted to evaluate 109 RP patients (32 PRP and 77 SRP) attending our Multidisciplinary Raynaud Clinics of the Clinical Immunology Unit at Centro Hospitalar do Porto in Portugal. We excluded from our study all patients with risk factors that could potentially interfere with flow-mediated dilatation (FMD): smokers, diabetics, with hyperlipidaemia, and with past history of myocardial infarction, as well as patients on bosentan treatment, due to possible interference with endothelin-1 levels (ET-1).
Controls and PRP patients were followed for 3-years to ensure no underlying secondary disease. All 77 SRP included patients had SSc based on 2013 classification criteria for SSc of American College of Rheumatology.10 A washout of the vasodilator drugs was done before inclusion in the study. Thirty-four healthy, sex/age matched, non-obese, without self-reported cardiovascular risk factors controls were invited to participate. No control subject was on any vasoactive medication.
SRP SSc-associated patients were divided into two groups: DU group, that included 38 patients having an active ischemic ulcer at inclusion (34 women; mean age 52.7±14.8 years; range 14–75); and a 39 patients group with no history of DU until enrolment (38 women; mean age 53.2±10.3 years; range 30–79).
The institutional ethical review board of Centro Hospitalar do Porto approved this study. All subjects signed informed consent before inclusion in the study. Data were collected by analysis of clinical file data and by clinical interview.
MethodsAllen testAllen test was performed as follows: (1) instruct patient to clench his/her fist; (2) apply occlusive pressure to both ulnar and radial arteries by finger pressure; (3) confirm palm and finger blanching with the patient's hand relaxed; (4) release the occlusive pressure on ulnar artery; (5) positive test: if the hand flushes within 5–15s, this indicates that the ulnar artery has good blood flow and palmar arch is complete; negative test: if the hand does not flush within 5–15s, this indicates that ulnar circulation is inadequate with an incomplete palmar arch.
Flow-mediated dilatation (FMD)Ultrasounds scans were performed using a two-dimensional ultrasonography General Electric Logic 7 with a 9MHz Linear wideband multihertz imaging probe. Ultrasound images were recorded and analyzed for 3 consecutive end diastolic frames (onset of R wave) at 45–60s after cuff deflation. The inter-operator variability was 3.6%.
Flow mediated dilatation of the brachial artery in the lower arm was evaluated following International Brachial Artery Reactivity Task Force Guidelines11 for the ultrasound assessment of brachial artery endothelial-dependent flow-mediated vasodilatation. Patients and controls (healthy subjects) were on overnight fasting for 12h before the ultrasound study was performed. The exams were performed in the morning, with patients being kept in a quiet temperature controlled room (22–24°C) for a preliminary 20-min rest. Vasoactive drugs were withheld for 10 half-lives. It was assured that patients did not exercise or ingest substances that could affect the response to ischemia like caffeine, vitamin C, tobacco or high-fat foods for 24h.
FMD was calculated as the percentage of change of the peak diameter in response to reactive hyperaemia in (FMD%=(peak diameter−baseline diameter/baseline diameter)×100).11
Vascular biomarkersVenous blood samples from fasting individuals were collected into a serum tube, and another tube containing sodium heparin (Vacuette, Greiner-Bio-One, Austria). Serum was allowed to clot at room temperature and then separated from cells within 60min, and stored at −70°C until analysis for asymmetric dimethylarginine (ADMA), endoglin, endostatin, vascular endothelial growth factor (VEGF-A).
ET-1 assessment: Plasma was centrifuged immediately in a refrigerated centrifuge and stored at −70°C until analysis for endothelin. Plasma endothelin was measured using a RIA assay (Euro-Diagnostics AG, Sweden). The resulting values are reported as pmol/ml.
ADMA assessment: Serum was allowed to clot at room temperature and then separated from cells within 60min and stored at −70°C before analysis for ADMA. Serum ADMA was measured using enzyme-linked immunosorbent assay (Immunodiagnostik AG, Germany). The resulting values are reported as μmol/L.
VEGF assessment: Serum VEGF-A was measured using enzyme-linked immunosorbent assay (IBL International GMBH, Germany). The resulting values were reported as pg/ml.
Endoglin and endostatin assessment: Serum endoglin and endostatin were measured using enzyme-linked immunosorbent assay (Uscn, Life Science Inc., Wuhan). The resulting values were reported as ng/ml.
Statistical analysisFor comparison of normally distributed scale variables, we used unpaired two-sided Student's t-test or analysis of variance (Anova). In these cases, data were described by mean±standard deviation (SD) followed by the minimal and the maximal values (range). Normal distribution was tested by Q-Q plots. In cases of non-normally distributed variables, we used non-parametric tests: Mann–Whitney and Kruskal Wallis tests and data were described by median followed by the interquartile interval (Q1–Q3), where Q1 represents the first quartile (corresponding to 25% of data) and Q3 represents the third quartile (corresponding to 75% of data). In Anova test, when the homogeneity of variance was not satisfied, we used the Welch test. For comparison of categorical variables, we used Chi-square or Fisher's exact probability test. A receiver operating characteristic (ROC) curve analysis was performed to obtain the predictive accuracy of FMD, MES score, ET-1, ADMA, VEGF, endostatin and endoglin. We considered p values <0.05 as significant. Data were analyzed using the SPSS software (v.22.0, SPSS, Chicago, IL).
ResultsThe demographic and clinical characteristics of the 143 subjects are described in Tables 1 and 2. No major differences were observed between SSc patients, PRP patients and control group regarding age, gender, mean arterial pressure and total cholesterol.
The demographic and clinical characteristics of the 143 subjects.
| Variables | PRP | SRP SSc-associated | Control | p-value | |
|---|---|---|---|---|---|
| DU | Non-DU | ||||
| Subjects, n | 32 | 38 | 39 | 34 | NA |
| Age (years), mean±SD | 49.9±12.5 | 52.7±14.8 | 53.2±10.3 | 47.1±10.96 | 0.137a |
| Gender Women, n (%) | 25 (78.1) | 34 (89.5) | 38 (97.4) | 29 (85.3) | 0.067b |
| Disease duration (years), median (Q1–Q3) | 15 | 10 | 10 | NA | 0.028*,c |
| Mean arterial pressure (mmHg), mean±SD | 87.6±5.6 | 87.9±6.04 | 88.3±6.4 | 86.7±6.8 | 0.75a |
| Total cholesterol (mg/dl), mean±SD | 188.8±8.8 | 191.3±9.0 | 187.1±12.1 | 190.6±7.6 | 0.242a |
| Allen test, positive (%) | 2 (6.3%) | 27 (71.1) | 7 (17.9) | 0 (0) | <0.001* |
RP: Raynaud phenomenon; PRP: primary; SRP: secondary RP; SSc: systemic sclerosis; DU: digital ulcer; NA: non applicable; SD: standard deviation; Q: quartile.
Comparison between SRP SSc-DU and SSc naïve DU groups.
| Variables | SSc DU | SSc naïve DU | p-value |
|---|---|---|---|
| Subjects, n | 38 | 39 | |
| Disease subset | 0.001*,a | ||
| Limited, n (%) | 26 (68.4) | 38 (97.4) | |
| Diffuse, n (%) | 12 (31.6) | 1 (2.6) | |
| Onset of 1st ulcer (years), median (Q1–Q2) | 5 (3–13.25) | NA | NA |
| Telangiectasias, positive, n (%) | 38 (100) | 27 (69.2) | <0.001*,a |
| Allen test, positive, n (%) | 27 (71.1) | 7 (17.9) | <0.001*,a |
| Autoantibodies | |||
| ACA, positive, n (%) | 22 (57.9) | 27 (69.2) | 0.301 |
| Scl-70, positive, n (%) | 12 (31.6) | 6 (15.4) | 0.093a |
| Anti-PM. Scl, positive, n (%) | 2 (5.3) | 5 (12.8) | 0.431b |
| Anti-RO 52, positive, n (%) | 20 (52.6) | 12 (30.8) | 0.052a |
| Anti-NOR, positive, n (%) | 0 (0) | 3 (7.7) | 0.240b |
| Anti-fibrilarin, positive, n (%) | 0 | 1 (2.6) | 1.000b |
| Anti U1 RNP, positive, n (%) | 2 (5.3) | 2 (5.1) | 1.000b |
| NVC pattern | |||
| Early, n (%) | 0 (0) | 13 (33.3) | <0.001a |
| Active, n (%) | 11 (28.9) | 22 (56.4) | |
| Late, n (%) | 27 (71.1) | 4 (10.3) |
SSc: systemic sclerosis; DU: digital ulcer; dcSSc: diffuse systemic sclerosis subset; lcSSc: limited systemic sclerosis subset; SRP: secondary Raynaud phenomenon; ACA: autoantibody anti-centromere; NVC: nailfold videocapillarosocopy; MES: microangiopathy evolution score; NA: no applicable.
Disease duration was significantly longer in PRP patients (median value: 15 years) compared to SRP SSc patients (median value: 10 years) (p=0.028). All PRP patients and controls had negative ANA and normal capillaroscopy.
Macrovascular diseaseMacrovascular disease was evaluated by clinical and hemodynamic parameters. Only 6.3% of PRP patients had a positive Allen test. However it was positive in 71% of SRP SSc-associated patients with DU whilst only 18% in patients without DU (p<0.001).
Macrovascular ultrasound examination showed no difference in braquial artery diameter between groups (p=0.620). Primary RP (p<0.001), SRP non-DU (p=0.001) and SRP DU (p=0.002) had significantly decreased basal state PSV compared to control group. No differences were found between SRP patients with and without DU (p=0.989) (Table 3).
Comparison of variables investigated between SRP, PRP and controls at baseline.
| Variables | PRP (n=32) | SRP-SSc (n=77) | Control (n=40) | p-value |
|---|---|---|---|---|
| FMD %, mean±SD | 17.96±12.78 | 10.85±11.0 | 20.17±8.86 | <0.001*,b |
| PSV 60s after cuff deflation (cm/s), mean±SD | 177.69±26.69 | 165.35±53 | 199.77±32.93 | <0.001*,b |
| EDV 60s after cuff deflation (cm/s), mean±SD | 92.95±35.05 | 67.28±24.37 | 93.70±20.01 | <0.001*,b |
| RI, mean±SD | 0.47±0.23 | 0.51±0.18 | 0.43±0.08 | =0.034b |
| ET-1pmol/ml, median (Q1–Q3) | 7.53 (0.16–11.73) | 11.85 (7.42–17.23) | 2.48 (0.00–5.60) | <0.001*,a |
| ADMA, μmol/L, median (Q1–Q3) | 0.40 (0.37–0.49) | 0.49 (0.41–0.54) | 0.38 (0.32–0.43) | <0.001*,a |
| Endoglin ng/ml, median (Q1–Q3) | 0.52 (0.28–0.88) | 2.17 (1.27–4.21) | 0.28 (0.15–0.71) | <0.001*,a |
| Endostatin ng/ml, median (Q1–Q3) | 0.90 (0.38–1.43) | 0.51 (0.19–1.24) | 0.565 (0.35–0.77) | 0.268a |
| VEGF pg/ml, median (Q1–Q3) | 438.50 (269.26-854.00) | 290 (166.71–361.78) | 178.03 (101.27–222.10) | <0.001*,a |
SSc: systemic sclerosis; DU: digital ulcer; SRP: secondary Raynaud phenomenon, ET-1: endothelin-1; ADMA: asymmetric dimethylarginine; VEGF: vascular endothelial growth factor. FMD: flow mediated dilatation; Q; quartile; SD: standard deviation.
aKruskal Wallis.
*bAnova test: Statistical significance for a level of 5%.
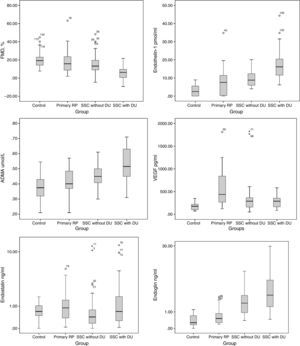
Flow-mediated dilatation at 60s after deflation was significantly lower in SRP patients (p<0.001). Patients with DU had significantly reduced FMD% (p<0.001) when compared to all other groups. No statistical differences were found between PRP and control groups (p=0.999) and between PRP and SRP SSc-associated non-DU (p=0.07). Fig. 1. No correlation was found between FMD and disease duration (R=0.41).
After 5min braquial artery occlusion, PRP and SRP had significant differences regarding EDV (p<0.001) and RI (p=0.007). PSV and EDV were significantly decreased in SRP group SSc-associated DU group (p<0.001). Table 3.
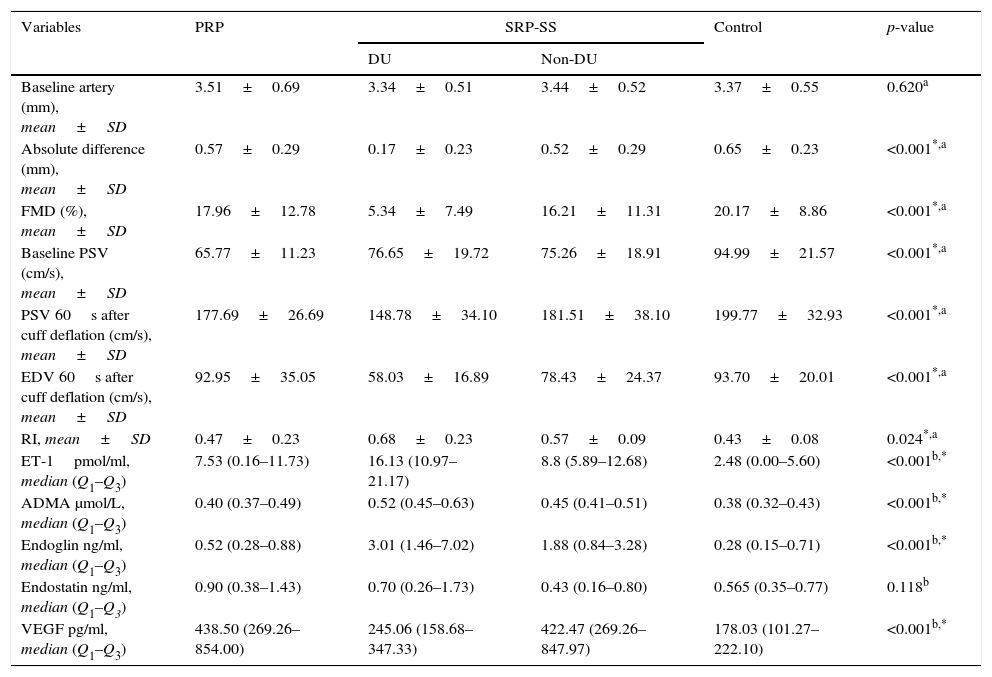
Vascular disease biomarkers Tables 3 and 4 and Fig. 1.
Comparison between PRP, SRP (with and without digital ulcers) and controls.
| Variables | PRP | SRP-SS | Control | p-value | |
|---|---|---|---|---|---|
| DU | Non-DU | ||||
| Baseline artery (mm), mean±SD | 3.51±0.69 | 3.34±0.51 | 3.44±0.52 | 3.37±0.55 | 0.620a |
| Absolute difference (mm), mean±SD | 0.57±0.29 | 0.17±0.23 | 0.52±0.29 | 0.65±0.23 | <0.001*,a |
| FMD (%), mean±SD | 17.96±12.78 | 5.34±7.49 | 16.21±11.31 | 20.17±8.86 | <0.001*,a |
| Baseline PSV (cm/s), mean±SD | 65.77±11.23 | 76.65±19.72 | 75.26±18.91 | 94.99±21.57 | <0.001*,a |
| PSV 60s after cuff deflation (cm/s), mean±SD | 177.69±26.69 | 148.78±34.10 | 181.51±38.10 | 199.77±32.93 | <0.001*,a |
| EDV 60s after cuff deflation (cm/s), mean±SD | 92.95±35.05 | 58.03±16.89 | 78.43±24.37 | 93.70±20.01 | <0.001*,a |
| RI, mean±SD | 0.47±0.23 | 0.68±0.23 | 0.57±0.09 | 0.43±0.08 | 0.024*,a |
| ET-1pmol/ml, median (Q1–Q3) | 7.53 (0.16–11.73) | 16.13 (10.97–21.17) | 8.8 (5.89–12.68) | 2.48 (0.00–5.60) | <0.001b,* |
| ADMA μmol/L, median (Q1–Q3) | 0.40 (0.37–0.49) | 0.52 (0.45–0.63) | 0.45 (0.41–0.51) | 0.38 (0.32–0.43) | <0.001b,* |
| Endoglin ng/ml, median (Q1–Q3) | 0.52 (0.28–0.88) | 3.01 (1.46–7.02) | 1.88 (0.84–3.28) | 0.28 (0.15–0.71) | <0.001b,* |
| Endostatin ng/ml, median (Q1–Q3) | 0.90 (0.38–1.43) | 0.70 (0.26–1.73) | 0.43 (0.16–0.80) | 0.565 (0.35–0.77) | 0.118b |
| VEGF pg/ml, median (Q1–Q3) | 438.50 (269.26–854.00) | 245.06 (158.68–347.33) | 422.47 (269.26–847.97) | 178.03 (101.27–222.10) | <0.001b,* |
RP: primary Raynaud phenomenon; SRP: secondary Raynaud phenomenon; SSc: systemic sclerosis; DU: digital ulcer; FMD: flow mediated dilatation; PSV: peak systolic velocity; EDV: end diastolic velocity; RI: resistive index. ET-1: endothelin-1; ADMA: asymmetric dimethylarginine; VEGF: vascular endothelial growth factor. FMD: flow mediated dilatation; Q; quartile.
ET-1 plasma levels were found to be significantly higher (p<0.001) in patients with both PRP and SRP SSc-associated compared with controls. A statistically significant difference for ET-1 plasma levels was observed between patients with PRP and SRP SSc-associated patients (p<0.001).
Among patients with SSc, ET-1 plasma levels were significantly higher (p<0.001) in patients with DU. No statistically significant difference for ET-1 plasma levels was observed between the PRP and SRP SSc patients without DU.
ADMAADMA serum levels were significantly higher in the SRP SSc-associated DU group (p<0.001). No significant differences were found between SRP SSc non-DU and PRP (p=0.757) and between PRP and controls (p=0.204).
VEGFSignificant differences were found in VEGF between PRP and SRP patients (p<0.001) and between PRP and control group patients (p<0.001). Lower plasma levels of VEGF were found in patients with fingertip digital ulcers (p<0.001). We found no difference when PRP was compared to SRP SSc non-DU group (p=0.099).
EndoglinAngiostatic serum endoglin levels were increased in SRP patients with active DU (p<0.001) and no significant difference was found between other groups.
EndostatinNo significant differences were found between groups (p=0.118). Comparing SRP with PRP no significant difference was found (p=0.302).
The AU-ROC (CI95%) of the macrovascular parameters and vascular biomarkers investigated associated to SRP were: FMD (AUC: 0.737 95%CI: 0.655–0.819); post-occlusion PSV (AUC: 0.681 95%CI: 0.593–0.768); post-occlusion EDV (AUC: 0.766 95%CI: 0.689–0.844); RI (AUC: 0.634 95%CI: 0.544–0.725), ET-1 (AUC: 0.826 95%CI: 0.758–0.895); ADMA (AUC: 0.754 95%CI: 0.675–0.832); VEGF (AUC: 0.508 95%CI: 0.410–0.606); endoglin (AUC: 0.914 95%CI: 0.870–0.959) and endostatin (AUC: 0.591 95%CI: 0.463–0.720).
DiscussionWe report here on the clinical and laboratory data regarding a large group of patients with diagnosis Raynaud's phenomenon. This is an observational cohort study of 109 RP patients that were divided into two subpopulations PRP and SRP, the latter in 2 groups (with or without previous ischemic peripheral lesions).
Clearly, our findings suggest that endothelial dysfunction suggested by increased serum levels of ET-1 as well a pro-angiogenic state due to increased serum levels of VEGF are already present in PRP and when comparing these patients with SRP SSc-associated without DU no major difference were found regarding the vascular biomarkers investigated. Thus, a new and useful information coming out of this investigation is that severe obliterative peripheral vasculopathy is present only in SRP patients with DU as expressed by the increased peripheral resistance, low FMD response to shear stress, decreased PSV and EDV and high RI mostly consequent of the EC injury with endothelial dysfunction associated to an impaired angiogenesis.
RP occurs when the balance of vascular tone is disturbed, favoring vasoconstriction. This endothelial activation and/or damage leads to reduce efficacy of vasodilators and/or overproduction of vasoconstrictors.4 Doubts persist whether there is overproduction of endothelium vasoconstrictor endothelin-1 (ET-1), underproduction of vasodilators such as nitric oxide (NO) and prostacyclin, or whether they are impaired in RP.4 Further complicating the role of NO, patients with SRP and SSc, have increased the plasma levels of an endogenous inhibitor of endothelial NOS—asymmetric dimethyl arginine— (ADMA) leading to reduced NO production.12
As a response to increase in shear stress, several vasodilators are released such as NO, prostaglandins and endothelium-derived hyperpolarizing factor.13 This response is commonly known as flow-mediated dilatation (FMD), and has been largely used for endothelium-dependent dysfunction assessment. NO is probably the major mediator of vasodilation and reduced NO bioavailability has been broadly accepted as a marker of endothelium dysfunction.14
In our cohort increased serum levels of ET-1 were present in PRP and SRP but only SRP SSc-associated patients with DU had significantly increased plasma levels of an endogenous inhibitor of endothelial NOS–ADMA. This favors early endothelial dysfunction with overproduction of vasoconstrictors (ET-1) even in PRP but only in severe SRP with peripheral vasculopathy is there an impaired inhibition of endothelial NOS. Furthermore endothelial dependent FMD was impaired in SRP, whilst PRP and control groups had similar response to shear stress.
Controversial results have been published regarding endothelial dysfunction assessment in SRP SSc patients. A systematic review and meta-analysis15 analyzed FMD assessment in SSc patients demonstrating that most of the studies (71%) assessing the FMD% found significantly lower brachial artery FMD% in SSc patients compared to controls. The lack of compensatory increase in blood flow to the ischemic stimulus may be due to endothelial dysfunction, reduced compliance, impaired distensability or increased arterial stiffness.16–18
Positive Allen test has been associated to RP and SSc.19 Occlusion of ulnar artery in SSc patients as a predictor of DU has been reported20 probably due to lack of compensatory flow of radial/ulnar artery and incomplete palmar arch. In this study patients with DU had more positive Allen tests compared to other groups favoring macrovascular disease in these patients.
Endothelial cell damage results in ischemia-reperfusion injury due to the ongoing pathological process, which inevitably evolves toward chronic underperfusion. Chronic hypoxia due to reduced blood flow is not compensated by efficient angiogenesis; even though elevated angiogenic biomarkers VEGF in SSc patients may be an attempt to induce neoangiogenesis and capillary neoformation. Yet, increased serum levels of angiostatic markers, such as endoglin, angiostatin or endostatin, may counteract this activity.12
SRP SSc-associated with DU patients expressed lower VEGF and increased angiostatic endoglin serum levels suggesting impaired vascular remodeling in response to the chronic ischemia. No significant differences were found when PRP and SRP SSc-associated patients with no peripheral lesions were compared.
ConclusionIn conclusion endothelial dysfunction and a pro-angiogenic stimulus are already present in patients with PRP. Macrovascular disease, increased peripheral resistance due to structural lesions and an impaired response to shear stress are characteristic of SRP, particularly in patients with peripheral ischemic lesions. SRP SSc-associated patients with DU overproduce endothelial dysfunction (ET-1 and ADMA) and angiostatic (endoglin) vascular biomarkers.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interest and source of fundingNone.