Prosthetic graft infection is a major complication of vascular surgery associated with high morbid-mortality rates. This retrospective non-randomized single center study evaluated our experience in the management of prosthetic vascular graft infections.
MethodsWe review the clinical files of patients who had vascular grafts implanted at our center between June 2007 and December 2011 and analysed the cases that developed Samson group 3, 4 and 5 infections until December 2012.
ResultsFrom June 2007 to December 2012, 18 consecutive patients (14 males, 4 females) with median age 70 years were admitted to our institution with the diagnosis of vascular graft infection accounting for an incidence of 3.8%. 50% of these infections were early infections and MRSA was the most prevalent pathogen. 44% of infections were due to infection of a femoro-popliteal bypass. Using Samson classification, 72% were group 4 and 5 infections. We performed graft preservation in one patient, graft excision without revascularization in 50% (nine) patients; Excision + insitu replacement in 39% (seven) patients; Excision + Extra-anatomic bypass in one patient. Our amputation rate was 55% and our related death rate was 16%.
ConclusionsOur amputation and mortality rates are according the published reviews. Besides allowing recognition of our reality this offers the opportunity to review diagnosis and therapeutic issues in prosthetic vascular graft infections. Each situation needs to be individualized as there is no consensus nor guiding algorithms about what should be the best medical treatment.
A infeção de enxertos protésicos vasculares é uma complicação grave da cirurgia vascular, cursando com altas taxas de morbimortalidade. Este estudo retrospetivo não randomizado, unicêntrico, avaliou a sua experiência na gestão de infeções de próteses vasculares.
MétodosFez-se uma revisão dos processos clínicos das revascularizações protésicas vasculares realizadas no nosso centro entre junho de 2007 e dezembro de 2011 e selecionaram-se aquelas que desenvolveram infeções do grupo 3, 4 e 5 da classificação de Samson até dezembro de 2012.
ResultadosDesde junho de 2007 a dezembro de 2012, 18 doentes (14 homens, 4 mulheres), com uma média de idade 70 anos, foram admitidos no nosso centro com o diagnóstico de infeções de próteses vasculares contribuindo para uma incidência de 3,8%. 50% das infeções foram precoces sendo o MRSA o patogéneo mais isolado. 44% das infeções deveram-se a infeção de conduto femoro-poplíteo. Usando a classificação de Samson, 72% foram infeções grupo 4 e 5. Realizámos preservação do enxerto num doente, excisão da prótese sem revascularização em 50% (nove) doentes; Excisão + substituição in situ em 39% (sete) doentes; Excisão + bypass extra-anatómico num doente. A nossa taxa de amputação foi de 55% e a nossa mortalidade relacionada foi de 16%.
ConclusõesAs taxas de amputação e mortalidade da série estão de acordo com as revisões publicadas previamente. Para além de permitir reconhecer a nossa realidade esta publicação oferece a oportunidade de rever o diagnóstico e questões terapêuticas relacionadas com infeções de enxertos vasculares protésicos. Cada situação deve ser individualizada, pois não há consenso nem protocolos estabelecidos sobre qual deve ser o tratamento de eleição.
Prosthetic vascular graft infections (PVGI) are a catastrophic event associated with high morbidity and mortality rates.1 It's incidence ranges between 1 and 6%. The death rate ranges between 15 to 75% with a rate of major amputation that may reach 70%.2 Staphylococcus species are the most commonly implicated causative organisms,3 with Staphylococcus aureus more likely in early infection and coagulase-negative staphylococci such as Staphylococcus epidermidis more likely in late infections.4 The diagnosis of vascular graft infections is usually made on the basis of clinical findings, supported by radiological and microbiological investigations. As for infections of implanted material, it is recommended to replace the vascular prosthesis so as to eradicate the infection while preserving or restoring arterial vascularization. Various replacement vascular materials (in situ or extra-anatomic) are available such as antibiotic or silver coated vascular prostheses, autologous or heterologous venous or arterial grafts.2 More recently, the trend has been to move away from graft removal to graft preservation with the use of vacuum assisted closure (VAC) devices, with or without muscle flap coverage more commonly employed.5 We examined our experience with infected prosthetic grafts after surgical bypass and the impact of postoperative graft infection on amputation rates and mortality.
Material and methodsIn order to summarize our centre's experience, we retrospectively reviewed the clinical files of vascular graft infections implanted at the department of Angiology and Vascular Surgery at Coimbra's University Hospital Center, from 01.06.2007 to 31.12.2011, and analysed the cases of PVGI identified until 31.12.2012. We defined a graft infection as clinical e laboratory evidence of infection associated with the presence of fluid directly communicating with the graft (in an imagiological or intra-operative view) or an exposed graft. We excluded arteriovenous and endovascular grafts; grafts implanted in another institution; bypass grafts performed for management of previous PVGI and infections categorized in Samson group 1 or 2. Once all the clinical files were identified, an individual detailed questionnaire was completed with the study data. Patient demographics, body mass index, comorbidities, indications for intervention, location of bypass, type of prosthetic material, case urgency, and previous ipsilateral bypass or percutaneous interventions were recorded as well as the timing of infection, diagnosis, bacteriology, treatment and outcome.
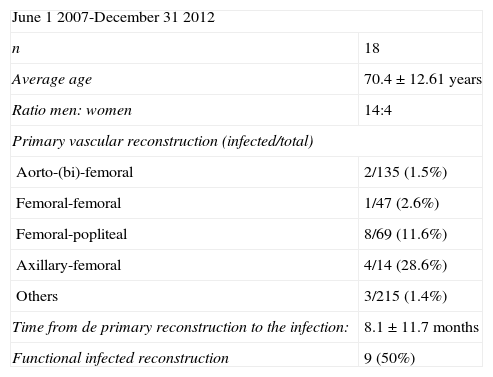
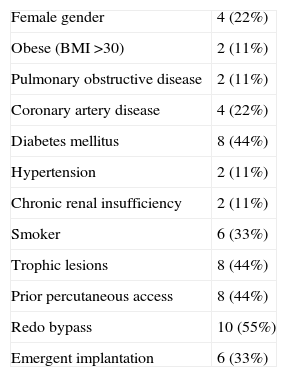
ResultsBetween 01.06.2007 and 31.12.2012, a total of 18 patients with PVGI that met the inclusion criteria were admitted to our department. During the study period 480 prosthetic grafts were implanted, accounting for a 3.8% incidence of PVGI (Tables 1 and 2). Subjects are ranged from 28 to 82 years (average age 70.4±12.61 years) and the male/ female ratio was 14:4. The most prevalent risk factors were Diabetes Mellitus (44%), tissue loss (44%), previous arterial puncture (44%) and previous ipsilateral incision (55%). Thirteen revascularization procedures were performed in ten patients before the implantation of the graft. Only two of these 13 revascularizations consisted in prosthetic material (occluded ABFP due to precarious outflow, supplemented with femoro-popliteal reconstruction). 33% of the grafts were implanted in an emergent setting. 55% of grafts were used for management of failed previous revascularization (Table 3) and for arterial occlusive disease in 28% (5/18). 22% (4/18) consisted of polyethylene terephthalate (Dacron) material and 78% of polytetrafluoroethylene (PTFE).
Demographic data.
| June 1 2007-December 31 2012 | |
| n | 18 |
| Average age | 70.4±12.61 years |
| Ratio men: women | 14:4 |
| Primary vascular reconstruction (infected/total) | |
| Aorto-(bi)-femoral | 2/135 (1.5%) |
| Femoral-femoral | 1/47 (2.6%) |
| Femoral-popliteal | 8/69 (11.6%) |
| Axillary-femoral | 4/14 (28.6%) |
| Others | 3/215 (1.4%) |
| Time from de primary reconstruction to the infection: | 8.1±11.7 months |
| Functional infected reconstruction | 9 (50%) |
Patient characteristics (No. (%)).
| Female gender | 4 (22%) |
| Obese (BMI >30) | 2 (11%) |
| Pulmonary obstructive disease | 2 (11%) |
| Coronary artery disease | 4 (22%) |
| Diabetes mellitus | 8 (44%) |
| Hypertension | 2 (11%) |
| Chronic renal insufficiency | 2 (11%) |
| Smoker | 6 (33%) |
| Trophic lesions | 8 (44%) |
| Prior percutaneous access | 8 (44%) |
| Redo bypass | 10 (55%) |
| Emergent implantation | 6 (33%) |
BMI, body mass index.
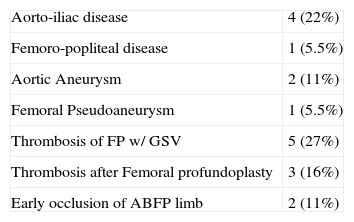
Indication for revascularization.
| Aorto-iliac disease | 4 (22%) |
| Femoro-popliteal disease | 1 (5.5%) |
| Aortic Aneurysm | 2 (11%) |
| Femoral Pseudoaneurysm | 1 (5.5%) |
| Thrombosis of FP w/ GSV | 5 (27%) |
| Thrombosis after Femoral profundoplasty | 3 (16%) |
| Early occlusion of ABFP limb | 2 (11%) |
ABFP, aorto-bi-femoral bypass; FP, femoro-popliteal; GSV, great saphenous vein.
After implantation of the graft, redo bypass was performed in a total of 33% (6/18) patients, of which, 3 during the same time hospitalization and 3 in a different hospitalization (interval of 30 to 275 days). Again these 3 procedures were performed due to thrombosis, with purpose of limb salvage. There was an 11% incidence of wound infection and 33% of surgical sutures dehiscence. Limbs underwent ulcer debridement/minor amputations in 33% of the cases all at the initial hospital stay.
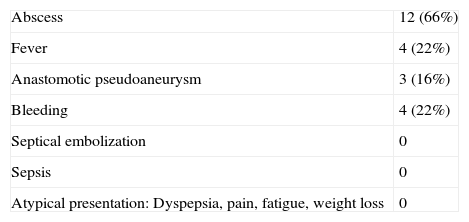
The diagnosis of PVGI was established by patient history, clinical examination (Table 4), laboratory tests (C-reactive protein, leucocytosis), microbiologic profile, ultrasonography (USG), computed tomography (CT). Using Samson classification, 6 cases were grade V, 7 cases grade IV, and 5 cases grade III.
The mean period from the primary intervention to the occurrence of infection was on average 8.1±11.7 months. In 9 cases it was an early infection (within the first 4 months). 44% (8) of infected grafts consisted of femoro-popliteal bypass. Most patients (72%) had an imagiological confirmation of infection (CT by 60%). Bacterial culture was available in 100% patients (28% from wound exsudate, 38% from intra-operative peri-graft liquid and 72% from culture of the graft). Staphylococcus organisms (Staphylococcus aureus (16%), Methicillin-resistant Staphylococcus aureus (50%)) were detected most often, followed by Gram negative organisms (Pseudomonas aeruginosa, Klebsiella, Morganella) in 33%, Enterococcus (16%) and anaerobic spp in 1 case. In 17% of the cases, a polymicrobial infection was present. No patient was treated with antibiotics before hospital admission due to symptoms of graft infection. All the patients were treated empirically with antibiotics after admission, and after bacteriology results, they were treated according to the sensitivity. The antibiotherapy was applied for the minimum period of 2-6 months (mean duration of intravenous antibiotics: 30.38 days).
At the time of admission, 50% of the grafts were patent. We performed preservation of the prosthesis with radical surgical surgical debridement in one patient (Samson 3 infected aorto-bifemoral bypass without involvement of the graft body), extirpation in 50% cases, in situ bypass with autologous vein in 22%, cadaveric vein in 22% and with graft in 5.5%. In the preserved graft we used local rotational muscle flap closure with Sartorius muscle. During the management of the infected bypass, each patient underwent a mean of 2.4 surgical procedures.
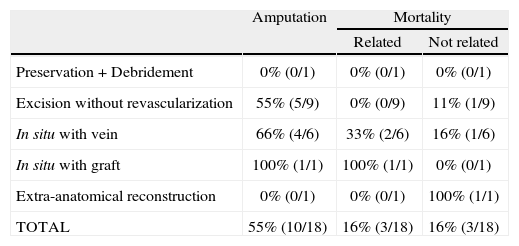
The mean follow-up was 2 years. We had to perform a major amputation in 55% of patients (Table 5). Two patients, who had an above-knee amputation, had recurrent infections requiring further debridement. The preserved graft was successfully salvaged. Some patients who required graft removal had no reconstructive options or the limbs were not salvageable at the time of presentation with PVGI. In the group that had a patent graft and underwent excision without revascularization (3 cases), 67% (2/3) had limb salvage. The global mortality was 33% (6 patients) – three cases of sepsis and three not related deaths (oral cancer, one acute myocardial infarction, one splenic infarction). Two of the three related deaths were Samson group 4 and the remaining Samson group 5. In the three fatalities MRSA was isolated (in two with co-associated Gram negative bacteria: Pseudomonas + Morganella multiresistent). 17% were lost to follow-up.
Treatment and major complications of the prosthetic vascular graft infections.
| Amputation | Mortality | ||
| Related | Not related | ||
| Preservation + Debridement | 0% (0/1) | 0% (0/1) | 0% (0/1) |
| Excision without revascularization | 55% (5/9) | 0% (0/9) | 11% (1/9) |
| In situ with vein | 66% (4/6) | 33% (2/6) | 16% (1/6) |
| In situ with graft | 100% (1/1) | 100% (1/1) | 0% (0/1) |
| Extra-anatomical reconstruction | 0% (0/1) | 0% (0/1) | 100% (1/1) |
| TOTAL | 55% (10/18) | 16% (3/18) | 16% (3/18) |
Prosthetic aortic graft infections represent a major diagnostic and therapeutic challenge.6 The leading cause of graft infection is contamination at the original surgical procedure. Emergency surgery, poor sterile technique, bowel injury during the operation, extensive lymphatic manipulation or injury during the dissection, extended preoperative hospital stay predisposing the patient to virulent pathogens and prolonged operating time are frequent predisposing factors for graft infections.7 The presence of an active infection at the time of the operation is predictive of Surgical Site Infections (SSIs) and prosthetic graft infection, most likely owing to contamination during the procedure or perioperative period. In our case series 44% of cases had trophic lesions in the limbs. Siracuse et al., in their retrospective study about prosthetic graft infections involving the femoral artery concluded that redo bypass, female gender, diabetes, and active infection at the time of bypass are associated with a higher risk for graft infection, which results in subsequent early major extremity amputation.5 A redo operation was also predictive of graft infection, likely secondary to scarring and poor vascular supply. On our series, 33% of the patients needed a redo revascularization after implantation of the prosthesis. Of these six patients, 5 (83%) ended up with limb loss.
According to Edwards et al., surgical wound complications (skin necrosis, hematoma, seroma e cellulites) are identified as primary predisposing factors in 33% of PVGI. More than one third of infections had previous wound complications.8 In our case the incidence of wound complications was 39% (7/18).
Another factor playing a role in aortic graft infections may be the type of prosthetic graft material used for repair. There is probably no significant difference in the incidence of infection of Dacron grafts compared with PTFE grafts. However, after infection develops, Dacron grafts may be more resistant to eradication of infection than PTFE grafts and Dacron may be more commonly associated with systemic sepsis and anastomotic disruption.7
The authors found a PVGI incidence of 3.8% in their cohort. But it had into account intracavitary prosthesis (149 interventions). If we exclude these procedures the adjusted incidence increases to 5.5%. Surprisingly the authors found that 30% of axillary-femoral conducts (4/14), a high incidence compared to literature (4.1%). In what concerns the four axillary-femoral grafts infections, it is known that subcutaneous tunnels increase the risk of PVGI. Also, this kind of vascular reconstruction is usually performed in poor functional capability patients. Three cases were perform secondary to aorto-iliac disease without physical conditions to perform an abdominal bypass and the forth case was after a femoral thromboendarterectomy that failed.
Avoidance of a prolonged preoperative hospital stay to minimize the development of skin flora resistant to commonly used antibiotics (i.e., hospital-acquired strains) is important.7,8 It is critical that intravenous antibiotics be administered within 1 hour of the skin incision. Administration of antibiotics before this time is of no benefit and may actually be harmful by predisposing to resistant antibiotics.7 They should be administrated during a prolonged surgery or when huge changes in vascular volume occur.8 Intravenous antibiotics after surgery is controversial. There are no sound data to prove that they should be continued more than 24 hours for routine cases.7 Longer duration of perioperative antibiotics (>48 hours) may be considered whenever patients present more than two risk factors for wound infection, including extremes of age, malnutrition, chronic illnesses such as diabetes, remote infections or prior irradiation of the surgical site.9
The most common bacteria cultured from infected grafts include Staphylococcus aureus, Staphylococcus epidermidis, diphtheroids, and gram-negative enteric organisms.7 In the past 2 decades, the incidence of MRSA has greatly increased, and it has become an important pathogen, contributing to 33% of vascular SSIs. In our series it represents a 50% contributing pathogen. Graft infections associated with negative culture results are caused by S. epidermidis or other coagulase-negative staphylococci, or by Candida species. Infections due to Gram-negative bacteria like E. coli, Pseudomonas, Klebsiella, Enterobacter and Proteus spp are very virulent9 compared with low virulence organisms such as coagulase negative Staphylococcus, Corynebacterium, or Propionibacterium species.10 In patients with a reduced immunity, serious mycotic graft infections may occur (Candida, Mycobacterium, Aspergillus).11
Clinical manifestations of PVGIs are variable and can range from an apparent superficial wound infection to a potentially life-threatening complication such as hematochezia due to an aortoenteric fistula. Patients presenting with an early-onset PVGI may only present with a superficial surgical wound infection. Systemic manifestations of infection such as fever, hypotension, and tachycardia are more frequent with more virulent organisms such as S. aureus and P. aeruginosa. However, other local complications such as perigraft abscess, anastomotic aneurysm, hemorrhage, and poor incorporation of the graft into surrounding tissue may be encountered. Lower extremity prosthetic vascular grafts may even be exposed to the outside. Moreover, graft occlusion and distal septic emboli may result in tissue ischemia and/or gangrene.10
Most cases of late PVGI have no general symptons.11 Local signs of a smoldering infection such as poor incorporation of the graft into surrounding tissues, sinus tract formation, graft occlusion, pseudoaneurysm formation, and aortoenteric fistula are more frequent.10
The basic diagnostic indicators, apart from clinical examination and patient's history, are increased levels of leucocytes, CRP, sedimentation and positive bacteriological culture from the site of the vascular infection and a positive hemoculture. TC should be the first image exam whenever there is a suspicion of aortic graft infections. USG is a first line exam to evaluate superficial grafts and inguinal tumefactions.12 Ultrasound guidance with findings of the accumulation of fluid around the vasculature reconstruction and false aneurysm in anastomosis, as well as CT and MRI have a high sensitivity and specificity for PVGI. Suspected PVGI on CT or MRI can be identified by a free prosthesis with no signs of healing in the surrounding tissue, by the accumulation of fluid with gas bubbles around the vascular reconstruction, tissue swelling around reconstruction and false anastomotic aneurysm. Using the mentioned examinations, in the absence of clinical manifestation, it is difficult to distinguish the early PVGI from post-operative changes, simple hematoma and the presence of gas after surgery,11 which can be a normal postsurgical finding up to 3 months after the procedure in cases of liquid collections and up to 1 week in case of air pockets.10 White blood cell scanning is an important complementary test to CT in ambiguous cases, such as in the early postoperative period, and may be more sensitive in detection of early graft infection.13
Four basic principles involved in the management of PVGI include 1) complete excision of all infected material; 2) extensive debridement of the infected and necrotic tissue in the perigraft area; 3) appropriate antimicrobial therapy based on identification of the causative organisms; and 4) restoration of the distal blood supply.7,10
Intraoperative tissue specimens should be submitted to the microbiology laboratory for bacterial (gram stain), fungal and mycobacterial stains and cultures.10 While awaiting the culture results, empiric broadspectrum antibiotic therapy that includes coverage for resistant gram negative and gram-positive organisms including methicillin resistant staphylococci should be initiated. Empiric use of carbapenems may be considered in institutions with a high prevalence of extended-spectrum b-lactamase–producing gram-negative organisms.10 In our series 77% (14/18) were treated empirically with vancomycin and in 50% of the cases there was an association with carbapenems (imipenem). Until recently daptomycin was not available in our institution. The characteristics of daptomycin (rapid and intense bactericidal action including on bacteria with low metabolism, diffusion in the biofilm) at strong doses make it a good agent for the treatment of VPIs.12 For cases where intra-abdominal graft infection from a gastrointestinal source is a consideration, empiric anaerobic coverage should also be added. Empiric antifungal or anti-mycobacterial coverage is generally not needed. In general, a minimum of 4 to 6 weeks of systemic antibiotic therapy is recommended10,11 and subsequent oral therapy for the period 3-6 months.10 For our statistical analysis, total duration of antibiotic therapy was not possible to collect because after the initial intravenous therapy there is a scarce information on medical records about ambulatory oral antibiotics as well as its total duration.
The surgical therapeutic options are: Graft preservation; Graft excision without revascularization; Excision + in-situ replacement; Excision + Extra-anatomic bypass.9 Success of graft or route salvage is often determined by the infective organism, with pseudomonas and MRSA infected grafts being particularly difficult to salvage.14 The current literature indicates Samson group 3 patients are most often considered for graft salvage and route salvage (in situ reconstruction)12,14 with partial or total preservation of graft. Patients limited to Samson group 4 and 5 normally need complete excision of the graft. The current literature suggests that MRSA-infected graft preservation should only be attempted with minor graft involvement. The high proportion of amputations is likely due to the prevalence of MRSA and the excision of the graft in a population with already threatened limbs.12,14 The rate of amputation in MRSA group was 66% (6/9) and the remaining 22% need aggressive tissue excision with cutaneous flaps.
Excision of the infected reconstruction with a large scale debridement is only possible in patients who, as a result of PVGI, have developed thrombosis of the reconstruction with sufficient collateral circulation. Even then it is associated with a high number of amputations.11
In situ reconstruction can be accomplished by using three different types of graft materials: autogenous vein grafts, cryopreserved or fresh arterial allografts and antibiotic-bonded prosthetic grafts. Arterial and vein grafts have the benefit of lower re-infection rates compared with prosthetic grafts. Vein grafts have the added advantage of higher long-term patency rates compared with the arterial grafts.10 On four patients we used cryopreserved veins: in two as a single option and the other two because of failed great saphenous vein bypass. 3 of these resulted in amputation and in the remaining we had to perform excision of the cadaveric vein due to inguinal dehiscence and exposition of the vascular role. For antibiotic bonded prosthetic vascular grafts, rifampicin is the most commonly used antibiotic to coat grafts. There is also a silver coated prosthesis which demonstrate a very good healing ability in tissue.11
Extra-anatomic bypass graft was traditionally considered the gold standard procedure because it avoids placing a new graft in a previously infected surgical bed. However, results of a systematic review in 2006 revealed that overall rate of reinfection, amputation, conduit failure, and mortality was slightly lower in patients who underwent in situ reconstruction compared with extra-anatomic bypass surgery.10 Staged procedure may be chosen in order to minimize a reinfection of the new vascular reconstruction. Some authors prefer the simultaneous procedure as some clinical results did not support this approach.11
There are several limitations to our study. It is a single center retrospective review, in a short period of time (June 2007 and December 2011). There was no standardization for the choice of conduit; rather, it was surgeon dependent and based on personal preference, which could cause selection bias because patients who are viewed as high risk may have more vein options exhausted before using a prosthesis.
Either way the authors can conclude that graft infections can assume a catastrophic development with serious sequelae such as limb loss and can be lethal. In summary, therapeutic procedures in PVGI must not be generalized, as every patient with a PVGI requires a strictly individual approach in order to select the best therapeutic procedure.
Ethical disclousuresProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data.The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent.The authors declare that no patient data appear in this article.
The authors have no conflicts of interest to declare.