Hepatitis delta virus infection occurs as acute coinfection or as superinfection in patients with preexisting chronic hepatitis B. Chronic hepatitis delta leads to more severe disease than chronic hepatitis B, with more rapid progression of fibrosis and increased risk of hepatocelullar carcinoma.
Case reportWe report a case of hepatocelullar carcinoma 5 years after spontaneous clearance of Hepatitis B surface antigen in a patient with previous chronic hepatitis delta. He had been diagnosed with acute hepatitis delta superinfection 30 years ago which evolved to chronic delta infection and subsequently development of liver cirrhosis. Despite no specific antiviral treatment, he lost HBsAg persistently with later regression of cirrhosis.
ConclusionsIn patients with cirrhosis due to chronic hepatitis delta who cleared HBsAg with improvement of liver fibrosis by non invasive techniques, it remains unknown how long hepatocelullar carcinoma surveillance has to be maintained.
Hepatitis delta virus (HDV) infection only occurs in Hepatitis B surface antigen (HBsAg) carriers, either as acute coinfection or as superinfection in patients with pre-existing chronic hepatitis B virus (HBV) infection. Chronic hepatitis delta (CHD) leads to more severe disease than HBV monoinfection, with more rapid progression of fibrosis and increase risk of hepatocelullar carcinoma (HCC).1 Clearance of both hepatitis delta and B infection is achieved by HBsAg loss, either spontaneously or after pegylated Interferon therapy (PegIFN), although the rates are very low.2 HCC surveillance in patients with liver cirrhosis is recommended. However, in those who cleared CHD and cirrhosis has regressed, it is unknown how long HCC surveillance has to be maintained. Here, we report a case of HCC 5 years after spontaneous clearance of HBsAg.
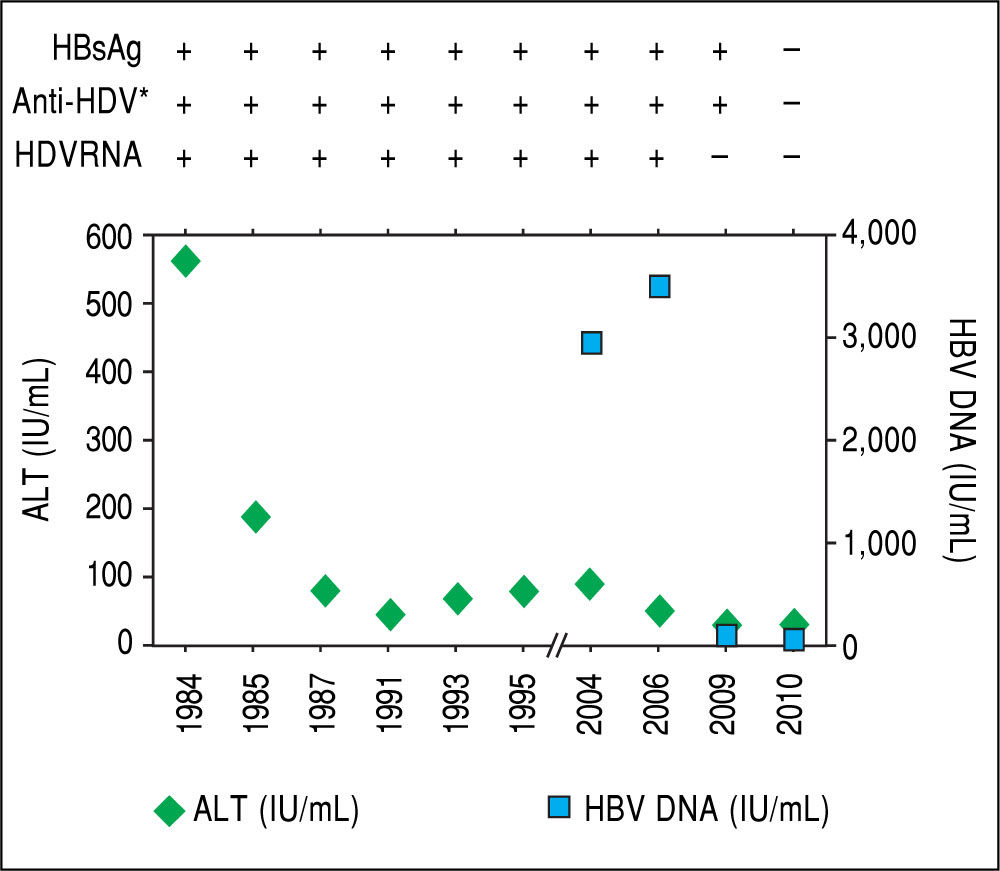
Case ReportA 53 year-old Caucasian man consulted to hospital in February 2015 for worsening asthenia, anorexia and weight loss over the previous 3 months. He was born in Barcelona. He worked as engineer. He referred occasional intake of alcohol during the weekends. He denied history of drug abuse. In April 1984 when the patient was 22 years old, he presented an icteric acute hepatitis with alanine aminotransferase (ALT) levels of 568 IU/mL and bilirubin of 4.7 mg/dL. At that moment, IgM antibody against hepatitis B core antigen (anti-HBc) and hepatitis B e antigen (HBeAg) were negative and HBsAg, anti-HBe and IgG anti-HDV were positive. He was diagnosed with acute hepatitis delta superinfection, in spite he was previously unaware of being an HBsAg carrier. The only remarkable antecedent was a dental procedure 2 months before. As resumed in the table, after acute infection ALT level decreased but remained intermittently elevated and HDV RNA was persistently detectable by spot hybridation method.3 HBV genotype was D. One year later, a liver biopsy showed chronic active hepatitis. In 1989 another biopsy revealed signs of cirrhosis with detection of delta antigen in the liver. Treatment with Interferon was proposed but the patient refused and he was lost to follow-up until 2004. Both anti-HCV and antibodies against human immunodeficiency virus (HIV) were negative. Direct sequencing by Sanger method showed a large deletion on the core promoter region. In 2010, without any specific therapy, HDV RNA became undetectable, HBsAg was cleared and seroconverted to anti-HBs, although low levels of HBV replication persisted (HBV DNA < 50 IU/ mL) (Figure 1). An abdominal ultrasound performed in 2013 showed a homogeneous liver parenchyma, liver stiffness measured by transient elastography was 5 kPa and HBsAg and HDV RNA remained undetectable and ALT and platelet count normal. Afterwards the patient discontinued follow-up.
After acute hepatitis due to HDV superinfection, ALT levels de creased although intermittent elevations were documented during follow-up. In 2004, when the patient started monitoring, HBV DNA was quantified and levels remained below 20,000 UI/mL. In 2010, without any specific treatment, HDV RNA became undetectable, and spontaneous HBsAg sero-clearance was documented. * IgG anti-HDV.
At admission to hospital in February 2015 physical examination was normal. Blood tests showed normal levels of platelets 249 x 10E9/L and increase alpha-fetoprotein levels (3620 IU/mL) and a computed tomography scan revealed multiple liver lesions suggestive of HCC, with a maximum diameter of 9 cm. A liver biopsy confirmed the diagnosis of HCC. HBsAg, HIV and anti-HCV remained negative. Treatment with Sorafenib was initiated with good tolerance. However, disease progressed and bone metastases were detected.
DiscussionWe presented a case of spontaneous resolution of chronic hepatitis D and B infection in a male with liver cirrhosis that developed an HCC on the follow-up. Spontaneous clearance of HDV in Delta superinfection cases is exceptional. Therapy with PegIFN is associated with HDV RNA clearance in approximately 25% of cases after 6 months of follow up, but late relapses can occur and the concept of sustained virological response cannot apply for HDV as it is done in Hepatitis C.2 Recent cohort studies following CHD patients for more than 28 years showed high cumulative rates of hepatic decompensation and mortality with low spontaneous rates of HBsAg clearance. In a study carried out in Italy including 299 patients with CHD, the spontaneous annual rate of seroconversion from HBsAg to anti-HBs was 0.25%.4 In fact, 2 patients with cirrhosis developed a HCC after HBsAg loss, but less than 2 years after seroconversion. Moreover, Asian HBV-infected patients who lost HBsAg developed HCC in 2.4% of cases, although the majority of cases were patients older than 50 years at seroconversion.5 This study showed a relationship between age of patients at the time of HBsAg loss and the risk of developing HCC, being the majority of cases in subjects older than 50 years at seroconversion.5 Our patient showed regression of cirrhosis evaluated by transient elastography, clearance of HBsAg at age of 48 and normal platelets levels, suggesting a very low risk of HCC.
Three large cohorts from Italy, Spain and Germany regarding the outcome of CHD patients have been published and the rate of spontaneous clearance of HBsAg was 1% (2/188), 7% (11/158) and 7.4% (10/136) respectively.6-8 In these series, no cases of HCC were reported in patients who cleared HBsAg.
Liver fibrosis was previously thought to be irreversible, but recent evidence shows that if the stimulus for chronic liver tissue damage is removed, early cirrhosis can be reversed. Data from chronically HBV-infected patients with long-term virological suppression under Tenofovir disoproxil fumarate therapy revealed regression of liver fibrosis and reversion of cirrhosis in 51% and 74% of patients, respectively.9 The guidelines recommended surveillance of HCC if cirrhosis has been developed before spontaneous or treatment HBsAg clearance because patients remains at risk of HCC. Currently, it is well-known that regression of cirrhosis is possible just by suppression of viral replication, even without HBsAg clearance. In both Caucasian and Asian HBV-infected patients the viral control by nucleos(t)ide analogues has been related with dramatic decrease of HCC incidence.10,11 This opens the door to try to score the risk of developing HCC after viral suppression or HBsAg clearance in clinical practice.
In conclusion, we present a well documented case of multifocal HCC diagnosed 5 years after spontaneously HBsAg loss in a patient with resolved CHD. This case highlights the importance of developing scores of HCC risk that allows individualization of HCC surveillance in patients with a functional cure of hepatitis B and D.
Abbreviations- •
ALT: alanine aminotransferase.
- •
CHD: chronic hepatitis delta.
- •
HBeAg: hepatitis B e antigen.
- •
HBsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HDV: hepatitis delta virus.
- •
HIV: human immunodeficiency virus.
- •
IgM anti-HBc: IgM antibody against hepatitis B core antigen.
- •
PegIFN: pegylated interferon therapy.
Maria Buti and Mar Riveiro-Barciela have received research grants from Gilead Sciences Europe. Maria Buti has served as advisor to Gilead, Bristol Myers Squibb and Novartis. The authors declare that they have received no funding for this manuscript.
AcknowledgementsWriting support was provided by Fidelma Greaves.