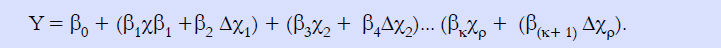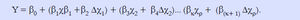Introduction and aim. Accurately predicting the prognosis of individual patient is crucial in the management of ACLF. We aimed to establish a specific prognostic model for HBV-related ACLF patients treated with nucleoside analog (NA).
Material and methods. We prospectively collected 205 ACLF cases diagnosed according to the APASL criteria. A dynamic prognostic model based on APASL criteria was established and validated. To demonstrate that the model is also applicable to those within EASL criteria, we divided the patients into two groups: met APASL criteria only (group A, n = 123); met both APASL and EASL criteria (group B, n = 82). Its prognostic accuracy was also compared with chronic liver failure-sequential organ failure assessment (CLIF-SOFA) score in group B.
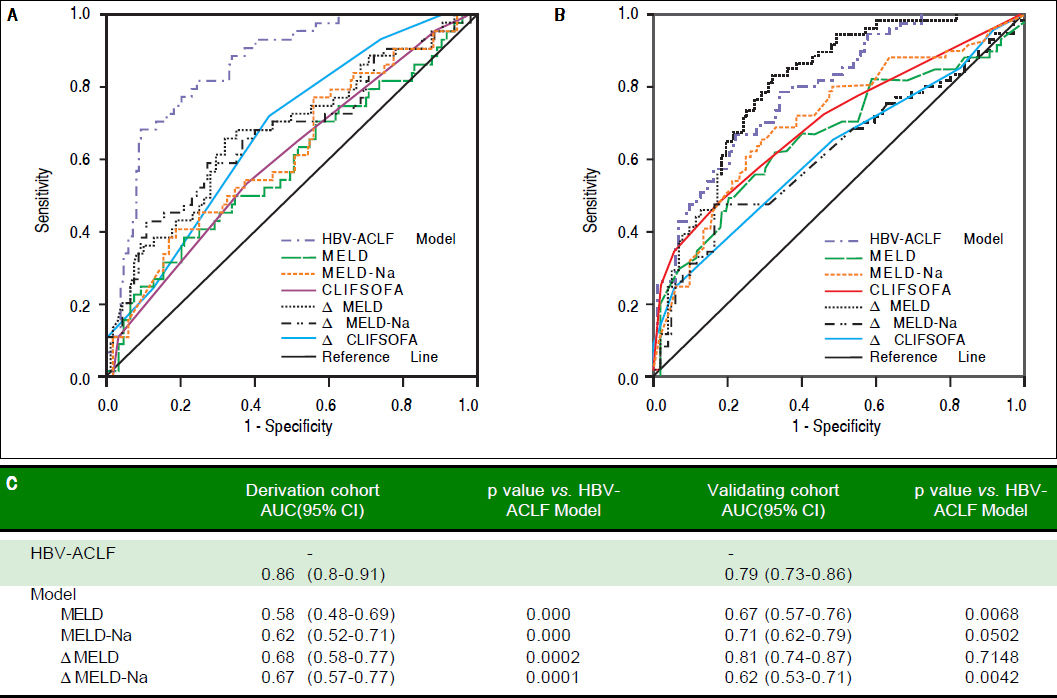
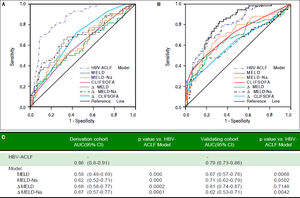
Results. The model is: R = 0.94 x Bilirubin + 0.53 x evolution of Bilirubin - 0.45 x PT-A - 0.22 x evolution in PT-A -0.1 x PLT + 10 x anti-HBe. The area under receiver operating characteristic curve (AUC) of the model for predicting 90-day mortality was 0.86, which was significantly higher than that of model for end stage liver disease(MELD), MELD-Na, CLIF-SOFA, ΔMELD (7d) and ΔMELD-Na (7d), ACLIF- SOFA(7d) (all p < 0.01). The AUC of our model in the validation group was 0.79 which was superior to MELD (0.45) CLIF-SOFA (0.53) score in group B patients (p < 0.01).
Conclusion. In conclusion, the model was superior to the conventional methods in predicting the outcomes of patients with HBV related ACLF treated with NA. It is the first description of a novel prognostic model using consecutive data in patients with HBV-induced acute-on-chronic liver failure (ACLF) treated by nucleoside analogs.
Acute on chronic liver failure (ACLF) is an acute decompensation of chronic liver disease, which is characterized by high mortality.1 Liver transplantation is the most effective lifesaving treatment.2 It is very important to predict the prognosis in order to select proper transplantation candidate.
There are currently four kinds of models to evaluate the severity and prognosis of patients with severe liver disease:3
- 1.
Liver-specific models such as Child-Turcotte -Pugh Score (CTP), Model for End-Stage Liver Disease (MELD) and Kings’ college hospital (KCH) criteria.
- 2.
General scoring systems such as simplified acute physiology score (SAPS II) and acute physiology and chronic health evaluation II (APACHE II)) and
- 3.
Organ failure models such as organ system failure score (OSF), sequential organ failure assessment score (SOFA). Recently, a chronic liver failure-sequential organ failure assessment (CLIF-SOFA) model was developed by EASL to evaluate the prognosis of ACLF.
- 4.
Some scores were specially developed for the different causes of severe liver dysfunction, such as the Maddrey score for severe alcoholic hepatitis.
Hepatitis B causes about 80% of ACLF in China.4 Many scores were developed for this specific type of ACLF. But none of them have been universally accepted. Majority of the previous studies did not account for the therapeutic effect of nucleoside analog (NA).5–7 In addition, the prognostic model based on a single time point may not suitable for ACLF which progresses rapidly. Based on the reasons above, the present study aimed to evaluate the prognostic factors of HBV-related ACLF patients treated with NA. We focused on the therapeutic response and the patient’s prognosis.
Materials and MethodsStudy populationThe protocol was approved by the Beijing Youan Hospital ethics committee and conformed to the guidelines of the Helsinki Declaration. All patients provided written informed consent. If the patient had encephalopathy or was unable to provide consent, it was obtained from the next-of-kin. We prospectively enrolled 205 HBsAg positive patients with ACLF from 2009 to 2011 in 7 hospitals as model group. The hospitals were Beijing Youan Hospital, Capital Medical University, The Ninth Hospital of Nanchang,The Second People’s Hospital of Fuyang, Hepatobilary Hospital ofJilin Province, The First Teaching Hospital of Xinjiang Medical University,The First Affiliated Hospital of Lanzhou University, The Sixth People’s Hospital of Kaifeng. All patients conformed to the guidelines of Helsinki Declaration and provided a written informed consent. A total of 165 patients hospitalized as ACLF from 2011 to 2015 in 3 hospitals (Beijing Youan Hospital, Capital Medical University, The Ninth Hospital of Nanchang and the Second People’s Hospital of Fuyang) were used as a validation group.
The inclusion criteria were: met the APASL ACLF criteria; HBsAg positive; treated by entecavir or lamivudine; age older than 18 years. Exclusion criteria: patients with a past history of decompensated cirrhosis; other hepatitis viral infection such as HCV, other insulting factors such as alcoholism and surgery. Patients with malignancy, pregnancy and HIV-AIDS were also excluded.
Treatment and follow upThe medical treatments included: nutritional support (25-30kcal/kg/d, enteral or parenteral), treatment of complications such as ascites, hepatic encephalopathy, infection and hepatorenal syndrome. One of the HRS patients underwent hemodialysis. Antiviral therapy included entecavir (Baraclude®, Bristol-Myers Squibb, Shanghai, China) 0.5-1 mg/d and lamivudine (Heptodin®, Glaxo-SmithKline, Suzhou, China) 100 mg/d. Two patients had liver transplantation. Ascites was defined as follows:
- •
Grade 1. Mild, only visible on ultrasound and CT.
- •
Grade 2. Detectable with flank bulging and shifting dullness.
- •
Grade 3. Directly visible, confirmed with the fluid wave/thrill test.8
Diagnostic criteria of spontaneous bacterial peritonitis included:9 ascites fluid neutrophil count > 250/mL or positive ascitic fluid bacterial culture; ascites fluid neutrophil count < 250/mL but clinical suspicion of infection, such as fever, abdominal rigidity and increased serum WBC and/or neutrophils Hepatic encephalopathy was classified according to the West Haven Criteria.10
Data collection included demographics, basic diseases, precipitating factors, complications, viral tests, liver function, abdominal ultrasound, chest X-ray or computed tomography.
After enrolment, the patients were followed up once a week for the first month, then once every other week for 90 days.
Statistical analysisStatistical analysis was performed with the SPSS 13.0 software for windows (Chicago, IL, USA). All the parametric data were expressed as mean ± SD and differences between two groups were assessed by a Student t-test; nonparametric data were expressed as median (range) and differences between two groups were assessed by a Wilcoxon Rank sum test. For the data expressed as percentages, the differences between two groups were assessed by a χ2 test. Univariate analysis was performed for quantitative and qualitative data. All variables with a p value < 0.05 in the univariate analysis were selected for multivariate analysis to obtain the prognostic factors. The area under Receiver Operating Characteristic (ROC) curve was expressed as point (95% confidence interval).
We used a dynamic logistic regression model to capture the baseline predictors at admission and their changes at day 7 after admission. The area under the ROC curve (AUC) of the prognostic model was expressed as point (95% confidence interval). The model was specified as formula 1.
Y = 1 if the patient die within 90 days, y = 0 otherwise. Model comparison and selection was based on Delong test for statistical significance between AUC of different competing models.
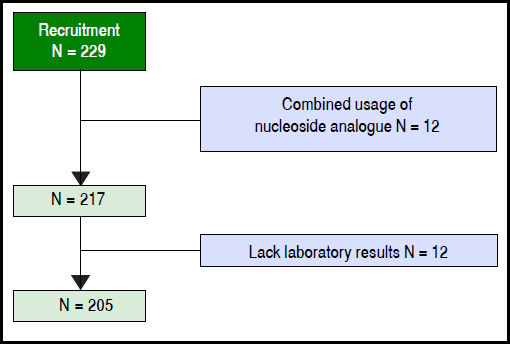
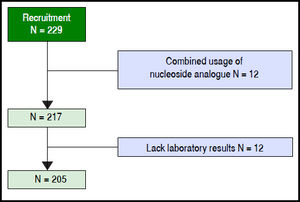
ResultsAmong 229 patients selected, 12 were ruled out because of combined usage of nucleoside analogue, 12 were ruled out because of the lack of key laboratory data. A total of 205 patients were included in the model group (Figure 1). The observation endpoint was death, liver transplantation or finish 90-day follow up. The mortality rates were 16.6% at 28 days and 23.9% at 90 days after enrollment. The median survival time of the deceased patients was 57 days (5-90) days.
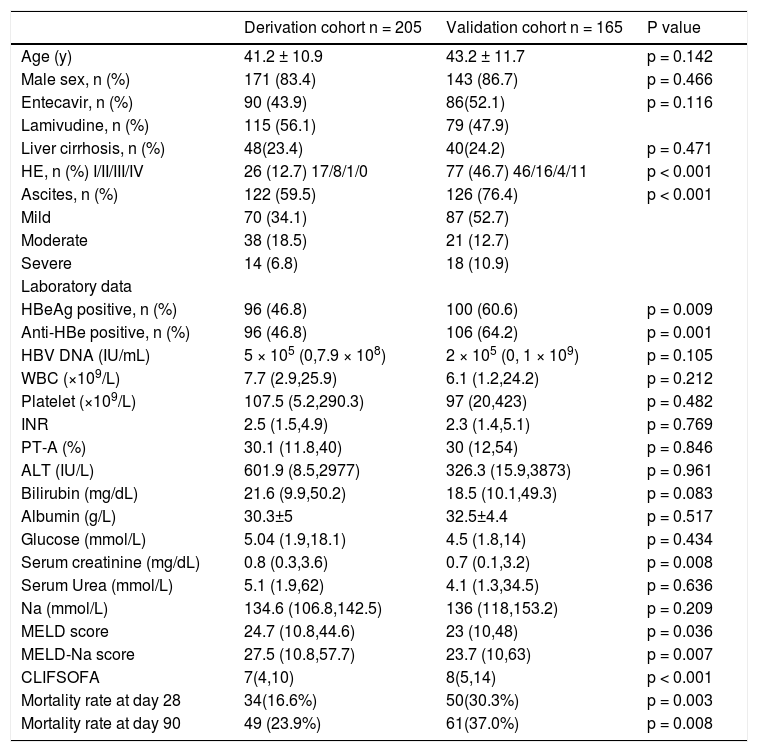
The clinical data were listed in table 1. Eighty three percent of the patients were male, the median HBV-DNA.
Demographic, clinical and biochemical features at enrollment of derivation and validation cohorts.
| Derivation cohort n = 205 | Validation cohort n = 165 | P value | |
|---|---|---|---|
| Age (y) | 41.2 ± 10.9 | 43.2 ± 11.7 | p = 0.142 |
| Male sex, n (%) | 171 (83.4) | 143 (86.7) | p = 0.466 |
| Entecavir, n (%) | 90 (43.9) | 86(52.1) | p = 0.116 |
| Lamivudine, n (%) | 115 (56.1) | 79 (47.9) | |
| Liver cirrhosis, n (%) | 48(23.4) | 40(24.2) | p = 0.471 |
| HE, n (%) I/II/III/IV | 26 (12.7) 17/8/1/0 | 77 (46.7) 46/16/4/11 | p < 0.001 |
| Ascites, n (%) | 122 (59.5) | 126 (76.4) | p < 0.001 |
| Mild | 70 (34.1) | 87 (52.7) | |
| Moderate | 38 (18.5) | 21 (12.7) | |
| Severe | 14 (6.8) | 18 (10.9) | |
| Laboratory data | |||
| HBeAg positive, n (%) | 96 (46.8) | 100 (60.6) | p = 0.009 |
| Anti-HBe positive, n (%) | 96 (46.8) | 106 (64.2) | p = 0.001 |
| HBV DNA (IU/mL) | 5 × 105 (0,7.9 × 108) | 2 × 105 (0, 1 × 109) | p = 0.105 |
| WBC (×109/L) | 7.7 (2.9,25.9) | 6.1 (1.2,24.2) | p = 0.212 |
| Platelet (×109/L) | 107.5 (5.2,290.3) | 97 (20,423) | p = 0.482 |
| INR | 2.5 (1.5,4.9) | 2.3 (1.4,5.1) | p = 0.769 |
| PT-A (%) | 30.1 (11.8,40) | 30 (12,54) | p = 0.846 |
| ALT (IU/L) | 601.9 (8.5,2977) | 326.3 (15.9,3873) | p = 0.961 |
| Bilirubin (mg/dL) | 21.6 (9.9,50.2) | 18.5 (10.1,49.3) | p = 0.083 |
| Albumin (g/L) | 30.3±5 | 32.5±4.4 | p = 0.517 |
| Glucose (mmol/L) | 5.04 (1.9,18.1) | 4.5 (1.8,14) | p = 0.434 |
| Serum creatinine (mg/dL) | 0.8 (0.3,3.6) | 0.7 (0.1,3.2) | p = 0.008 |
| Serum Urea (mmol/L) | 5.1 (1.9,62) | 4.1 (1.3,34.5) | p = 0.636 |
| Na (mmol/L) | 134.6 (106.8,142.5) | 136 (118,153.2) | p = 0.209 |
| MELD score | 24.7 (10.8,44.6) | 23 (10,48) | p = 0.036 |
| MELD-Na score | 27.5 (10.8,57.7) | 23.7 (10,63) | p = 0.007 |
| CLIFSOFA | 7(4,10) | 8(5,14) | p < 0.001 |
| Mortality rate at day 28 | 34(16.6%) | 50(30.3%) | p = 0.003 |
| Mortality rate at day 90 | 49 (23.9%) | 61(37.0%) | p = 0.008 |
HE: hepatic encephalopathy. WBC: white blood cell. INR: international normalized ratio. PT-A: prothrombin activity. ALT: alanine aminotransferase. MELD: model of end-stage liver disease. CLIFSOFA: chronic liver failure-sequential organ failure assessment. All the parametric data were expressed as mean ± SD and nonparametric data were expressed as median (maximum, minimum).
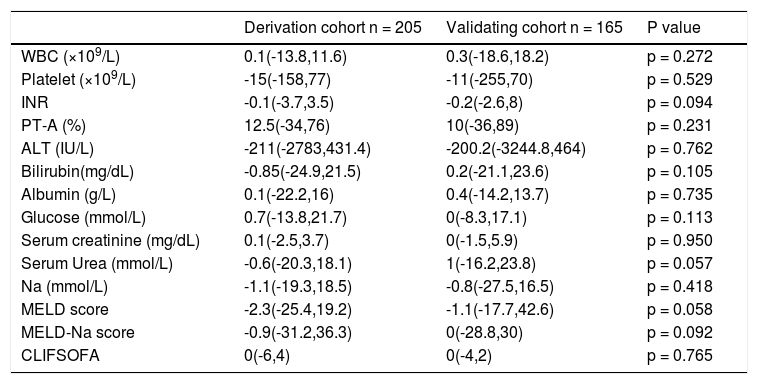
DNA was 5 × 105 (0, 7.9 x 108) (IU/mL), 46.8% of the patients were anti-HBe positive, 12.7% had hepatic encephalopathy at the time of enrollment. 59.5% had ascites, 1.5% had hepatorenal syndrome and 3.4% had upper gastrointestinal bleeding. The median MELD score was 24.7(10.8-44.6); the median MELD-Na score was 27.5(10.8-57.7). Ninety patients were treated with entecavir and 115 with lamivudine. One hundred and ninety-two cases had been given NAs before admission to the 7 hospitals. The others were given at admission. Since the mortality rates were similar (at 90 days between entecavir and lamivudine treated patients (22.2% v1s. 25.2%, p = 0.618), we did not compare further between these two groups. The change of variables after 7-day treatment was listed in table 2.
Change of variables at day 7 of derivation and validation cohort.
| Derivation cohort n = 205 | Validating cohort n = 165 | P value | |
|---|---|---|---|
| WBC (×109/L) | 0.1(-13.8,11.6) | 0.3(-18.6,18.2) | p = 0.272 |
| Platelet (×109/L) | -15(-158,77) | -11(-255,70) | p = 0.529 |
| INR | -0.1(-3.7,3.5) | -0.2(-2.6,8) | p = 0.094 |
| PT-A (%) | 12.5(-34,76) | 10(-36,89) | p = 0.231 |
| ALT (IU/L) | -211(-2783,431.4) | -200.2(-3244.8,464) | p = 0.762 |
| Bilirubin(mg/dL) | -0.85(-24.9,21.5) | 0.2(-21.1,23.6) | p = 0.105 |
| Albumin (g/L) | 0.1(-22.2,16) | 0.4(-14.2,13.7) | p = 0.735 |
| Glucose (mmol/L) | 0.7(-13.8,21.7) | 0(-8.3,17.1) | p = 0.113 |
| Serum creatinine (mg/dL) | 0.1(-2.5,3.7) | 0(-1.5,5.9) | p = 0.950 |
| Serum Urea (mmol/L) | -0.6(-20.3,18.1) | 1(-16.2,23.8) | p = 0.057 |
| Na (mmol/L) | -1.1(-19.3,18.5) | -0.8(-27.5,16.5) | p = 0.418 |
| MELD score | -2.3(-25.4,19.2) | -1.1(-17.7,42.6) | p = 0.058 |
| MELD-Na score | -0.9(-31.2,36.3) | 0(-28.8,30) | p = 0.092 |
| CLIFSOFA | 0(-6,4) | 0(-4,2) | p = 0.765 |
WBC: white blood cell. INR: international normalized ratio. PT-A: prothrombin activity. ALT: alanine aminotransferase. MELD: model of end-stage liver disease. CLIFSOFA: chronic liver failure-sequential organ failure assessment. All the nonparametric data were expressed as median (maximum, minimum).
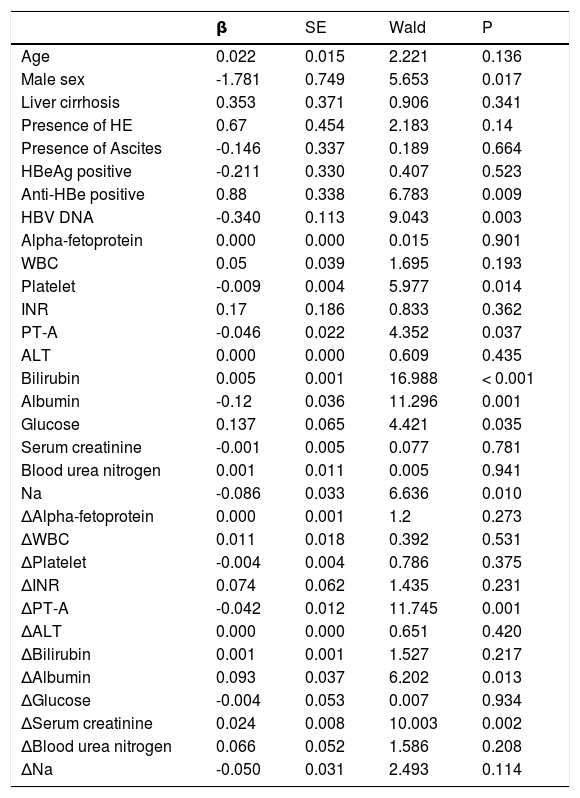
Univariate analysis showed (Table 3) that male, antiHBe positive, higher HBV DNA level, lower platelet, lower PT-A, higher bilirubin, lower serum albumin, lower serum glucose and lower serum sodium were all indicators of poor prognosis. Physicians routinely estimated the prognosis based on the baseline status of patients. However, the patients’ response to the treatment was also very important. Our univariate analysis showed that after 7-day treatment, the difference of PT-A, serum albumin and serum creatinine were also independent prognostic indicators.
Univariate analysis of factors.
| β | SE | Wald | P | |
|---|---|---|---|---|
| Age | 0.022 | 0.015 | 2.221 | 0.136 |
| Male sex | -1.781 | 0.749 | 5.653 | 0.017 |
| Liver cirrhosis | 0.353 | 0.371 | 0.906 | 0.341 |
| Presence of HE | 0.67 | 0.454 | 2.183 | 0.14 |
| Presence of Ascites | -0.146 | 0.337 | 0.189 | 0.664 |
| HBeAg positive | -0.211 | 0.330 | 0.407 | 0.523 |
| Anti-HBe positive | 0.88 | 0.338 | 6.783 | 0.009 |
| HBV DNA | -0.340 | 0.113 | 9.043 | 0.003 |
| Alpha-fetoprotein | 0.000 | 0.000 | 0.015 | 0.901 |
| WBC | 0.05 | 0.039 | 1.695 | 0.193 |
| Platelet | -0.009 | 0.004 | 5.977 | 0.014 |
| INR | 0.17 | 0.186 | 0.833 | 0.362 |
| PT-A | -0.046 | 0.022 | 4.352 | 0.037 |
| ALT | 0.000 | 0.000 | 0.609 | 0.435 |
| Bilirubin | 0.005 | 0.001 | 16.988 | < 0.001 |
| Albumin | -0.12 | 0.036 | 11.296 | 0.001 |
| Glucose | 0.137 | 0.065 | 4.421 | 0.035 |
| Serum creatinine | -0.001 | 0.005 | 0.077 | 0.781 |
| Blood urea nitrogen | 0.001 | 0.011 | 0.005 | 0.941 |
| Na | -0.086 | 0.033 | 6.636 | 0.010 |
| ΔAlpha-fetoprotein | 0.000 | 0.001 | 1.2 | 0.273 |
| ΔWBC | 0.011 | 0.018 | 0.392 | 0.531 |
| ΔPlatelet | -0.004 | 0.004 | 0.786 | 0.375 |
| ΔINR | 0.074 | 0.062 | 1.435 | 0.231 |
| ΔPT-A | -0.042 | 0.012 | 11.745 | 0.001 |
| ΔALT | 0.000 | 0.000 | 0.651 | 0.420 |
| ΔBilirubin | 0.001 | 0.001 | 1.527 | 0.217 |
| ΔAlbumin | 0.093 | 0.037 | 6.202 | 0.013 |
| ΔGlucose | -0.004 | 0.053 | 0.007 | 0.934 |
| ΔSerum creatinine | 0.024 | 0.008 | 10.003 | 0.002 |
| ΔBlood urea nitrogen | 0.066 | 0.052 | 1.586 | 0.208 |
| ΔNa | -0.050 | 0.031 | 2.493 | 0.114 |
HE: hepatic encephalopathy. WBC: white blood cell. INR. international normalized ratio. PT-A: prothrombin activity. ALT: alanine aminotransferase. MELD: model of end-stage liver disease.
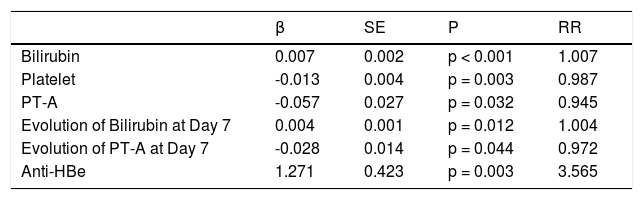
Multivariate analysis included the following indicators in the model (Table 4): baseline PT-A (%), the alteration at day 7; baseline bilirubin (mg/dL), the change at day 7; platelet (x109/L) and anti-HBe status (positive = 1, negative = 0) at baseline. Our dynamic prognostic model was in formula 2.
Multivariate analysis of variables.
| β | SE | P | RR | |
|---|---|---|---|---|
| Bilirubin | 0.007 | 0.002 | p < 0.001 | 1.007 |
| Platelet | -0.013 | 0.004 | p = 0.003 | 0.987 |
| PT-A | -0.057 | 0.027 | p = 0.032 | 0.945 |
| Evolution of Bilirubin at Day 7 | 0.004 | 0.001 | p = 0.012 | 1.004 |
| Evolution of PT-A at Day 7 | -0.028 | 0.014 | p = 0.044 | 0.972 |
| Anti-HBe | 1.271 | 0.423 | p = 0.003 | 3.565 |
PT-A: prothrombin activity.
AUC of this model was 0.856 (Figure 2A). If we set the cut off line to -0.73, the sensitivity was 67.3%, specificity was 91%. For example: if the baseline bilirubin was 25 mg/dL, PT-A was 20%, platelet was 30 × 109/L, anti-HBe was negative; 7 days later, PT-A decreased to 15%, bilirubin increased to 35 mg/dL, the calculated R = 0.77, there was a great probability that the patient will die. In comparison, if the baseline was the same, 7 days later after hospitalization, the patient’s PT-A increased to 25%, bilirubin decreased to15 mg/dL, R value should be -0.88, the patient had great probability to survive.
Comparison of the prognostic accuracy of HBV related acute-on-chronic liver failure dynamic model and other prognostic models. A. Derivation group. B. Validation group. ACLF: acute on chronic liver failure. MELD: model of end-stage liver stage. C. Comparison of AUCs between HBV-ACLF dynamic model and other prognostic models. AUC: The area under receiver operating characteristic curve.
To simplify the model formula, we multiplied it by 7.87 and derived the much simpler formula which was called HBV-ACLF dynamic model Formula 3.
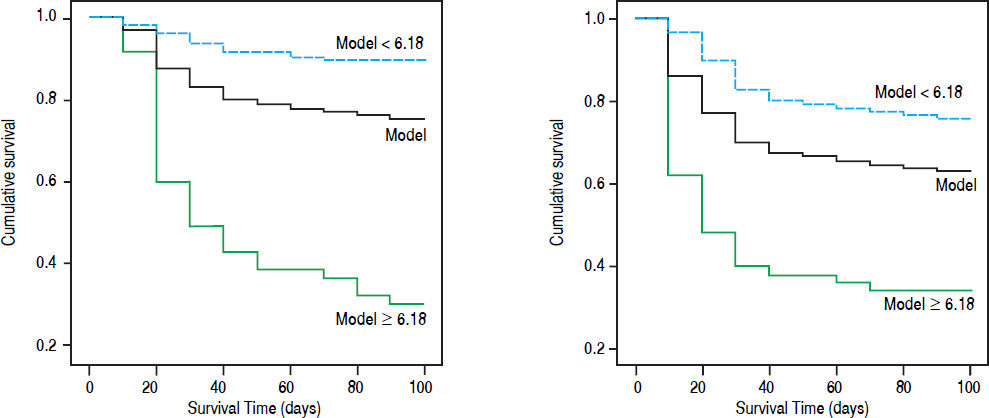
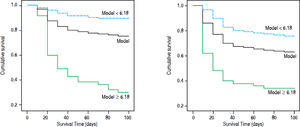
Bilirubin was mg/dL, PT-A was expressed as %, PLT as × 109/L, anti-HBe negative counts 0 and positive counts 1. Cutoff value was 6.18. The 90-day mortality rate in our cohort with R < 6.18 was 10.1% (16/158); this rate was significantly lower than that of patients with R > 6.18 (33/47, 70.2%, χ2 = 71.9, p = 0.000) (Figure 3A).
Survival curve according to cutoff of HBV related acute-on-chronic liver failure model. A. Model group. B. Validation group. The yellow line: the total accumulation survival curve; the blue line: the accumulation survival curve of patients with R < 6.18; green line: the accumulation survival curve of patients with R ≥ 6.18.
This model also applied to the patients who only have baseline values, for example, the patients within 7 days after admission, the changes of bilirubin and PT-A were 0, the AUC was 0.801 (0.737, 0.866). But the model drawn from baseline was not as accurate as the HBV-ACLF dynamic model (χ2 = 5.30, p = 0.0213).
Some special situationsThe applicability of HBV-ACLF dynamic model in patients with different MELD scoresThe cut off value was 23.9 when we use MELD to predict mortality. We therefore divided the patients into two groups according to their MELD score. The AUC in group with MELD score above and below 23.9 was 0.833 and 0.841, respectively (χ2 = 0.15, p = 0.6949). This indicated that HBV-ACLF dynamic model was applicable to predict patients’ outcomes in any ACLF patients regardless of severity as estimated by MELD score.
The impact of anti-HBe on the modelThe mortality rate was 32.3% in patients with anti-HBe positive in model group and 16.5% in those with anti-HBe negative (p = 0.008). Anti-HBe status did not affect the predictive accuracy of HBV-ACLF dynamic model (AUC in anti-HBe positive was 0.789 and negative, 0.89, χ2 = 2.92, p = 0.0872), the corresponding values of AUC in validation group were 0.856 vs. 0.878 (χ2 = 0.05, p = 0.8158).
Comparison between HBV-ACLF dynamic model and other modelsThe AUC values corresponding with MELD, ΔMELD-Na, CLIF-SOFA, ΔMELD (7d), ΔMELD-Na (7d) and CLIF-SOFA (7d)were 0.58, 0.62, 0.60, 0.68, 0.67 and 0.68, which were significantly lower than that of the HBV-ACLF dynamic model (all p < 0.001, figure 2A).
Validation of HBV-ACLF dynamic modelThe baseline variables and their changes at day 7 in validation group were listed in table 1 and 2. Compared with model group, the proportion of patients with HE, ascites and Anti-HBe positive were higher; while levels of creatinine, MELD and MELD-Na were lower. The changes of the values at day 7 were not significantly different between the derivation group and validation group. But the 28-day and 90-day mortality rates (30.3% and 37.0%) of validation group were significantly higher than those of derivation group. The AUC of the model was 0.79 (0.73-0.86) in validation group, which was higher than MELD (p = 0.0068) and ΔMELD-Na (p = 0.0042), CILF-SOFA (P = 0.022) and ΔCILF-SOFA (p = 0.0029); but not higher than MELD-Na (p = 0.0500) and MELD (p = 0.7148) (Figure 2B). When the cut off value was 6.18, the 90-day mortality of patients with R value below and above than 6.18 was 24.3%(28/115) and 66%(33/50) respectively (p = 0.000) (Figure 3B).
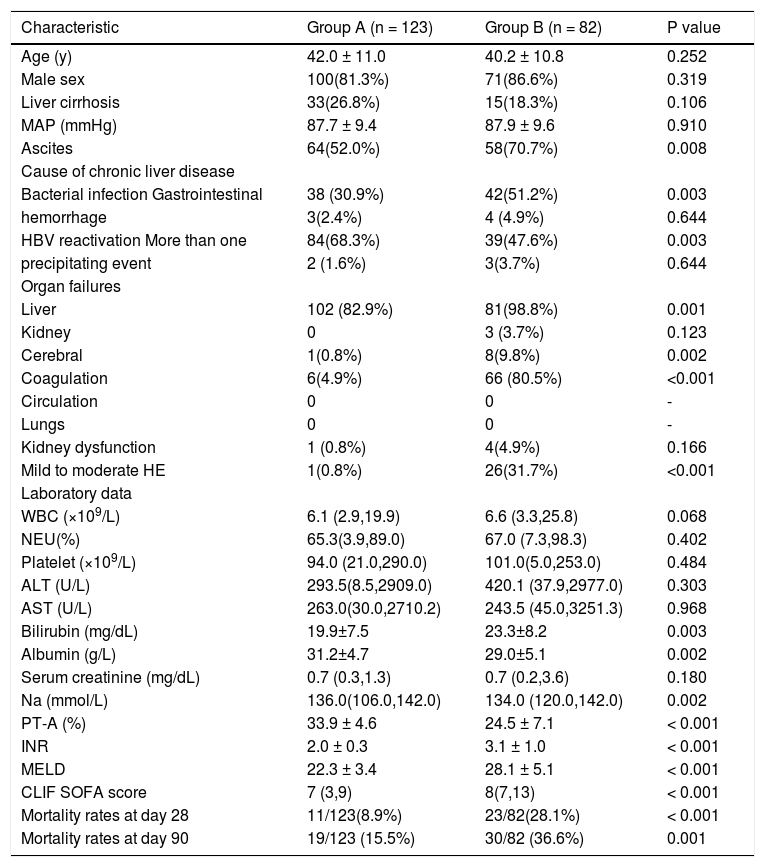
The prognostic ability of the model in group BThe patients were divided into group A (met APASL criteria only, n = 123) and group B (met both APASL and EASL criteria n = 82) (Table 5). Both the MELD score and the CLIF-SOFA score were significantly higher in group B and the 90-day mortality rate were 36.6% (group A 15.5%, p = 0.001). It’s obviously that group B patients were more serious than group A.
Characteristics of patients in group A and B at enrolment.
| Characteristic | Group A (n = 123) | Group B (n = 82) | P value |
|---|---|---|---|
| Age (y) | 42.0 ± 11.0 | 40.2 ± 10.8 | 0.252 |
| Male sex | 100(81.3%) | 71(86.6%) | 0.319 |
| Liver cirrhosis | 33(26.8%) | 15(18.3%) | 0.106 |
| MAP (mmHg) | 87.7 ± 9.4 | 87.9 ± 9.6 | 0.910 |
| Ascites | 64(52.0%) | 58(70.7%) | 0.008 |
| Cause of chronic liver disease | |||
| Bacterial infection Gastrointestinal | 38 (30.9%) | 42(51.2%) | 0.003 |
| hemorrhage | 3(2.4%) | 4 (4.9%) | 0.644 |
| HBV reactivation More than one | 84(68.3%) | 39(47.6%) | 0.003 |
| precipitating event | 2 (1.6%) | 3(3.7%) | 0.644 |
| Organ failures | |||
| Liver | 102 (82.9%) | 81(98.8%) | 0.001 |
| Kidney | 0 | 3 (3.7%) | 0.123 |
| Cerebral | 1(0.8%) | 8(9.8%) | 0.002 |
| Coagulation | 6(4.9%) | 66 (80.5%) | <0.001 |
| Circulation | 0 | 0 | - |
| Lungs | 0 | 0 | - |
| Kidney dysfunction | 1 (0.8%) | 4(4.9%) | 0.166 |
| Mild to moderate HE | 1(0.8%) | 26(31.7%) | <0.001 |
| Laboratory data | |||
| WBC (×109/L) | 6.1 (2.9,19.9) | 6.6 (3.3,25.8) | 0.068 |
| NEU(%) | 65.3(3.9,89.0) | 67.0 (7.3,98.3) | 0.402 |
| Platelet (×109/L) | 94.0 (21.0,290.0) | 101.0(5.0,253.0) | 0.484 |
| ALT (U/L) | 293.5(8.5,2909.0) | 420.1 (37.9,2977.0) | 0.303 |
| AST (U/L) | 263.0(30.0,2710.2) | 243.5 (45.0,3251.3) | 0.968 |
| Bilirubin (mg/dL) | 19.9±7.5 | 23.3±8.2 | 0.003 |
| Albumin (g/L) | 31.2±4.7 | 29.0±5.1 | 0.002 |
| Serum creatinine (mg/dL) | 0.7 (0.3,1.3) | 0.7 (0.2,3.6) | 0.180 |
| Na (mmol/L) | 136.0(106.0,142.0) | 134.0 (120.0,142.0) | 0.002 |
| PT-A (%) | 33.9 ± 4.6 | 24.5 ± 7.1 | < 0.001 |
| INR | 2.0 ± 0.3 | 3.1 ± 1.0 | < 0.001 |
| MELD | 22.3 ± 3.4 | 28.1 ± 5.1 | < 0.001 |
| CLIF SOFA score | 7 (3,9) | 8(7,13) | < 0.001 |
| Mortality rates at day 28 | 11/123(8.9%) | 23/82(28.1%) | < 0.001 |
| Mortality rates at day 90 | 19/123 (15.5%) | 30/82 (36.6%) | 0.001 |
MAP: mean arterial pressure. HE: hepatic encephalopathy. WBC: white blood cell. NEU: neutrophilic granulocyte. ALT: alanine aminotransferase. AST: aspartate aminotransferase. INR: international normalized ratio. PT-A: prothrombin activity. MELD: model of end-stage liver disease. CLIF SOFA: chronic liver failure-sequential organ failure assessment. All the parametric data were expressed as mean ± SD and nonparametric data were expressed as median (maximum, minimum).
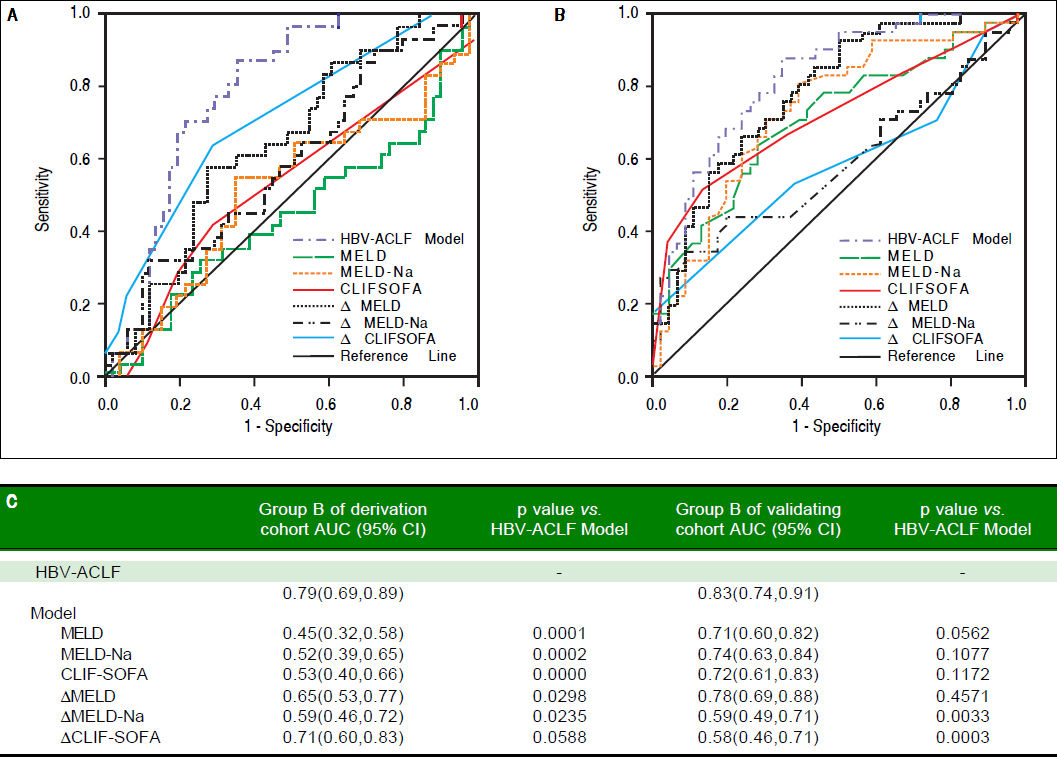
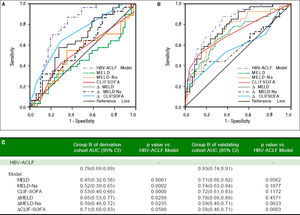
We compared our model to other models in group B who met both APASL and EASL criteria. AUC of our model, MELD, MELD-Na, CLIF-SOFA, ΔMELD, ΔMELD -Na, and ACLIF-SOFA was 0.79, 0.45, 0.52, 0.53, 0.65, 0.59 and 0.71, respecively. p value was all less than 0.05, except CLIF- SOFA (p = 0.0588) (Figure 4A).
Comparison of the prognostic accuracy of HBV related acute-on-chronic liver failure dynamic model and chronic liver failure-sequential organ failure assessment in group B. A. Group B in derivation group. B. Group B in validating group. ACLF: acute on chronic liver failure. MELD: model of end-stage liver stage. CLIF SOFA: chronic liver failure-sequential organ failure assessment. C. Comparison of AUCs between HBV-ACLF dynamic model and other prognostic models. AUC: The area under receiver operating characteristic curve.
In the group B of validation group, the AUC of our HBV-model was still the highest (0.83,0.74-0.91), and significantly higher than the AUC of MELD-Na (p = 0.0033) and ΔCLIF- SOFA (p =0.0003), but not higher than MELD (p = 0.0562),MELD-Na (p =0.1077), CLIFSOFA (p = 0.1172) and ΔMELD(p = 0.4571) (Figure 4B).
DiscussionThere are ~300 million HBV chronic carriers worldwide, with 75% of them in the Asia-Pacific area. Chronic hepatitis B is the leading cause of liver disease-related mortality.11 Two prospective studies found that 15-37% of patients with HBV infection had spontaneous acute exacerbation within 4 years.12,13 Some of the patients had sudden onset of ACLF and the mortality rate for these patients was as high as 30%-70%.14-16 Liver transplantation is currently still the most reliable therapeutic modality.2 Selection of liver transplantation mainly relies on evaluation of prognosis. However, there is currently no ideal model to predict prognosis in patients with ACLF. The present prospective cohort study established a prognostic model for patients with HBV-related ACLF being treated with NAs. The main parameters were bilirubin and PT-A and their changes after 1 week, platelet count, and anti-HBe. The special aspects of this model were: a single cause of ACLF; the impact of antiviral therapy on prognosis was taken into account; and changes in liver function were used to reflect effectiveness of treatment.
The parameters in our model were different from those in the CLIF Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study, which was carried out by the European Association for the Study of the Liver and Chronic Liver Failure Consortium.1 The main reason may be the difference in patient selection. The diagnostic criteria for ACLF in the CANONIC study were based on the CLIFSOFA score, which was also the main prognostic factor. In contrast, PT-A and bilirubin abnormalities were the main parameters in the APASL criteria and were the main characteristics in our patients. In our cohort only 8(3.9%) patients had renal dysfunction at admission, and other organ failure, such as pulmonary failure, was also rare. The main parameters in our model were therefore PT-A and bilirubin. Furthermore, 60.3% of patients in the CANONIC study had alcoholic hepatitis. Severe alcoholic hepatitis caused an increase in white blood cell (WBC) count, therefore, WBC count was also an important prognostic factor. The present study showed that the average WBC count was only 7.7 × 109/L and was not correlated with prognosis. The different diagnostic criteria and cause of ACLF between the CANONIC and present studies were the main reason for inclusion of different prognostic parameters in the two models. The CLIF-SOFA score system was not applicable to patients with HBV-related ACLF.
Many studies17–19 showed that both ETV and LAM could improve the survival rate of HBV-related ACLF patients, and the effectiveness were similar.20 This is the same as our statistics. The mortality rates were 22.2% in entecavir treated patients and 25.2% in lamivudine treated cases in 90 days (p = 0.618).
One of the features of HBV-related ACLF was that the status of HBV infection has an impact on prognosis. The present study showed that patients who were positive for anti-HBe had worse prognosis. The reasons might be as follows:
- •
These patients had a relatively longer clinical course when compared to anti-HBe negative patients.21
- •
The rate of cirrhosis was lower in anti-HBe negative group than that in anti-HBe positive group.21
- •
One of the mechanisms of ACLF in patients with HBV infection is basal core promoter and precore mutation.22–24
With these mutations, HBeAg expression is decreased or becomes negative, while expression of HBV core antigen is increased, which in turn may induce a strong immune response and hepatocyte damage.
Platelet count was another prognostic factor in our study which agrees with previous studies of ACLF14,25–27 and ALF.28 Stravitz, et al. studied 1,598 cases of ALF and found that all patients have thrombocytopenia in the 1-7 days after admission. But platelets were significantly lower in 1 to 7 days after admission in patients with outcomes of death or liver transplantation than in those with spontaneous recovery. The decrease in platelets during days 1 to 7 after admission was proportional to the grade of hepatic encephalopathy, requirement for vasopressor and renal replacement therapy. They speculated that systemic inflammatory response syndrome (SIRS) activates platelets, yields microparticles, and results in clearance of platelet remnants and subsequent thrombocytopenia. This mechanism may also act in ACLF-induced thrombocytopenia. In ACLF patients with underlying chronic liver disease, the baseline platelet count is also associated with hypersplenism and decreased thrombopoietin synthesis.29 In addition, platelets contain abundant growth factors, such as serotonin,30 which are important promoters of liver regeneration.
Thrombocytopenia may thus contribute to insufficient liver regeneration. Furthermore, platelet consumption31 could be a result of micro-thrombosis in the liver and other organs which is often seen in sepsis patients. Microcirculatory dysfunction in the liver further causes hypoxia and hepatocyte necrosis.31 These speculations may explain the mechanism of thrombocytopenia in ACLF but need to be studied further.
ACLF is a disease that progresses quickly. The disease severity at the beginning and its progress both impact the prognosis. Ha and colleagues found that aggravation of hepatic encephalopathy and the increase of MELD score at day 7 are the indicators of poor prognosis.32 Huo, et al. have demonstrated that MELD was superior to the basic ΔMELD and CTP scores.33 Chamuleau, et al. have also confirmed that dynamic models are better than baseline ones in predicting prognosis of acute liver failure.34 Gustot, et al.35 found that assessment of ACLF patients at 3-7 days after admission was much better than assessment on admission when defining the need for liver transplantation. We compared our model to other models such as MELD, MELD-Na and CLIF-SOFA and found that even the changes at day 7 were not considered, our model is still better than or equal to others. In patients who initially present to a smaller hospital, they are usually transferred to a larger tertiary-care centre after days or weeks of onset. Thus the time of admission to the larger centre may not necessarily reflect the entire natural history.
Accordingly, dynamic rather than baseline assessment of ACLF may be more important in this rapidly progressive condition. Our study also showed that the AUC of the dynamic model was significantly higher than that of the baseline model.
In conclusion, our multicenter, prospective cohort study demonstrated that the dynamic model was superior to the baseline model in patients with HBV-induced ACLF undergoing treatment. Our dynamic model was superior to MELD and MELD-Na models. The present study may be helpful for clinicians in the management of patients with HBV-related ACLF treated with NAs.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
ALT: alanine aminotransferase. o
- •
APASL: Asian Pacific Association for Study of the Liver.
- •
AUC: area under the curve.
- •
CANONIC: chronic liver failure (CLIF) Acute-on-Chronic Liver Failure in cirrhosis.
- •
CLIF: chronic liver failure.
- •
CLIF-SOFA: chronic liver failure-sequential organ failure assessment.
- •
EASL: European Association for Study of the Liver.
- •
HBV: hepatitis B virus.
- •
HE: hepatic encephalopathy.
- •
INR: international normalized ratio.
- •
MAP: mean arterial pressure.
- •
MELD: model of end-stage liver disease.
- •
NEU: neutrophilic granulocyte.
- •
PT-A: prothrombin activity.
- •
WBC: white blood cell.
National Science and Technology Major Project (Grant No. 2016ZX10002008-04; PI: Zhongping Duan), the National Science and Technology Major Project (Grant No. 2016ZX10004906-014; PI: Jing Zhang), Beijing Municipal Science and Technology Project (Grant No. Z141100002114022; PI: Jing Zhang) Science and Technology New Star Programme of Beijing Fengtai District (PI: Wei Lin).