Oral presentations at the XVI National Congress of the Mexican Association of Hepatology
Más datosClinical trials demonstrated the efficacy and safety of direct antiviral agents (DAA) to treat hepatitis C virus infection (HCV) in patients with decompensated cirrhosis (DC); however, very few real-world studies have been reported in this group. Moreover, predictive factors for non-achieving sustained virological response (SVR) in DC are not entirely understood. Therefore, the aim was to verify the efficacy and safety of DAA and to identify risk factors for failure to achieve SVR in DC.
Materials and MethodsA real-world cohort study included HCV patients with DC [Child-Pugh B/C or A but with a history of previous clinical decompensation events like variceal bleeding (VB), hepatic encephalopathy (HE), or ascites]. All patients treated before transplant had MELD ≤ 20 and Child-Pugh ≤ 12 according to AASLD guidelines.
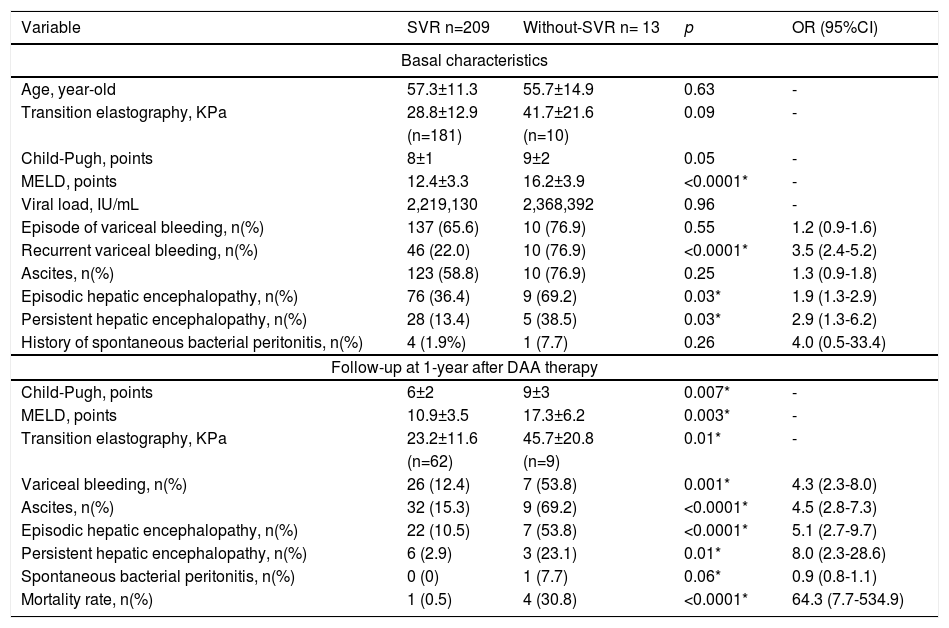
Results222 patients, 118 (53.2%) were women, mean age 57.2±11.5 year-old, 61 (27.5%) were treated with Sofosbuvir(SOF)/Ledipasvir, 152 (68.5%) with SOF/Velpatasvir, and 9 (4.1%) with SOF/Daclatasvir, 175 (78.8%) used also ribavirin, 209 (94.1%) achieved SVR, despite non-significant difference, Child-Pugh C patients had a suboptimal response (SVR < 95%): A 42/44 (95.5%), B 140/147 (95.2%), C 27/31 (87.0%), p=0.2. Related adverse events were fatigue 60 (27%), nausea 44 (19.8%), headache 43 (19.4%), non-severe peripheral edema 10 (4.5%), anasarca 4 (1.8%), jaundice 6 (2.7%), 3 hemolytic anemia (1.4%), 1 dermatosis (0.4%), congestive cardiac failure 2 (0.9%), need to suspend therapy due to liver-related adverse events 2 (0.9%) they also died. In those who achieved SVR, MELD improved (basal 12.4±3.3 vs. post-SVR 10.9±3.5; p<0.0001); but was worse in those without SVR (basal 16.2±3.9 vs. without-SVR 17.3±6.2; p=0.24). Times/year needing hospitalization for liver-related decompensation events were less frequent in those who achieved SVR (basal 1.7±1.3 vs. after SVR 0.4±0.7; p<0.0001) but remained without change in that without-SVR (basal 1.5±1.5 vs. after non-achieve SVR 2.2±1.9; p=0.04).
DiscussionA few real-world studies have been conducted in DC with hepatitis C. However, in the Mexican population, our study is the first that demonstrated in a real-world setting, similar to clinical trials, that DAA based on SOF and free of protease inhibitors (PIs) are effective and safe to cure HCV in DC. When to treat HCV before or after liver transplantation can be challenging. Classically, MELD >20 and Child-Pugh C >12 are related to non-SVR; however, our study also shows that additional clinical factors have a negative impact on SVR: history of recurrent VB and episodic and persistent HE; therefore, these criteria should also be considered to decide to treat previous or after liver transplantation. In addition, acute decompensation and mortality events are very high in those who do not achieve SVR.
ConclusionsSOF based on regimens without PIs are effective and safe in VHC with DC. Additional to classic criteria (MELD >20, Child-Pugh > 12), recurrent VB and HE are predictors of failure to achieve SVR in VHC with DC.
The authors declare that there is no conflict of interest.