In addition to the kidneys and lungs, the liver also plays an important role in the regulation of the Acid-Base Equilibrium (ABE). The involvement of the liver in the regulation of ABE is crucial because of its role in lactic acid metabolism, urea production and in protein homeostasis. The main acid-base imbalance that occurs in patients with liver cirrhosis is Respiratory Alkalosis (RAlk). Due to the fact that in these patients additional pathophysiological mechanisms that affect the ABE are present, other disorders may appear which compensate or enhance the primary disorder. Conventional ABE reading models fail to identify and assess the underlying disorders in patients with liver cirrhosis. This weakness of the classical models led to the creation of new physicochemical mathematical models that take into account all the known parameters that develop and affect the ABE. In addition to the RAlk, in patients with liver cirrhosis, metabolic alkalosis (due to hypoalbuminemia), hyponatremic metabolic acidosis, hyperchloremic metabolic acidosis, lactic acidosis and metabolic alkalosis due to urea metabolism are some of the pathophysiological mechanisms that affect the ABE.
Acid-Base Equilibrium (ABE) is an important parameter within an organism that determines the relationship between acids and bases produced daily by both endogenous (cellular) metabolism and (exogenous) acids and/or bases taken in through food. The acids of the organism are represented as the hydrogen ion (H+), while the bases by the bicarbonate radical (HCO3−).
The systems or organs of the body that are involved in the regulation of ABE are mainly the kidneys and the lungs. However, in recent years, it is increasingly recognized that the liver participates in regulation of ΑΒΕ, but not equivalently to the kidneys and lungs [1]. Knowledge of the role of the liver in the production of acids and/or bases or on the contrary in their reduction, contributes significantly to the understanding mainly of the mixed disorders of ABE [2]. For the understanding of ABE disorders in patients with liver diseases, theoretical data of ABE are presented in the next section.
1.1Principles of ABEIt is known that the serum concentration of H+ ([H+]) and consequently of the corresponding bases, is expressed through the pH index. For physiologically acceptable pH (7.4) the [H+] is 40nEq/L [3]. Acidemia is characterized by a condition where the pH is <7.36 and alkalaemia when the pH is >7.44.
To capture the balance of acids and bases in the blood, mathematical models based on the physicochemical properties of simple (water) and mixed solutions (such as blood) have been proposed from time to time. Of these models, the three main ones will be briefly outlined below.
- A.
The traditional ABE fixation model was proposed by Henderson-Hasselbalch who based on the chemical reaction of the decomposition of an acid [4]:
and considering the law of mass action (decomposition constant K), the state of equilibrium of acids and bases at a given moment is expressed by the equation:In the above model, H2CO3 is used as the acid, which when decomposed gives us a [H+] and a radical [HCO3−], so the previous equation is formed in:
However, in the above equation, the dissolved plasma CO2 is taken as acid since the plasma concentration of H2CO3 is minimal (at 37°C for every mmHg of PaCO2 the naturally dissolved CO2 in the blood is 0.03mEq/L). Therefore, the final equation of the Henderson-Hasselbalch mathematical model of ABE is:
Under normal conditions where PaCO2 is 40mmHg, [HCO3−] is 24mEq/L and the pKH2CO3=6.1 the pH=7.4.
- B.
The second physicochemical model of ABE was proposed by Stewart (1983) and it was based on the view that blood belongs to the category of solutions containing CO2 (the other three are: distilled water, strong ions, and weak bases) [5]. Thus, to calculate the [H+] in the blood and consequently the pH, at a given moment, the model of Strong Ions Difference (SID) was developed. This term expresses the difference between all strong cations and all strong anions. However, because strong anions are superior to strong cations, the SID is negative. Whereas the SID is considered to be an indicator representing the net unbalanced positive charge. The SID, according to the above model is calculated from the following equation:
The SID calculation from the above equation does not consider the role of weak acids such as albumin, phosphorus, and CO2 in the balance of electrical charges, so it is best to call the SID indicator apparent (SIDa). The role of weak acids is expressed through the calculation of the active SID (SID effective, SIDe) from the following Figge's equation [6]:
Theoretically, the difference between SIDa and SIDe should be 0 (equilibrium of electric charge in solution). If there is no electrical equilibrium, it is expressed as ion gap (SAG) [SIG = SIDa - SIDe] [7].
- C.
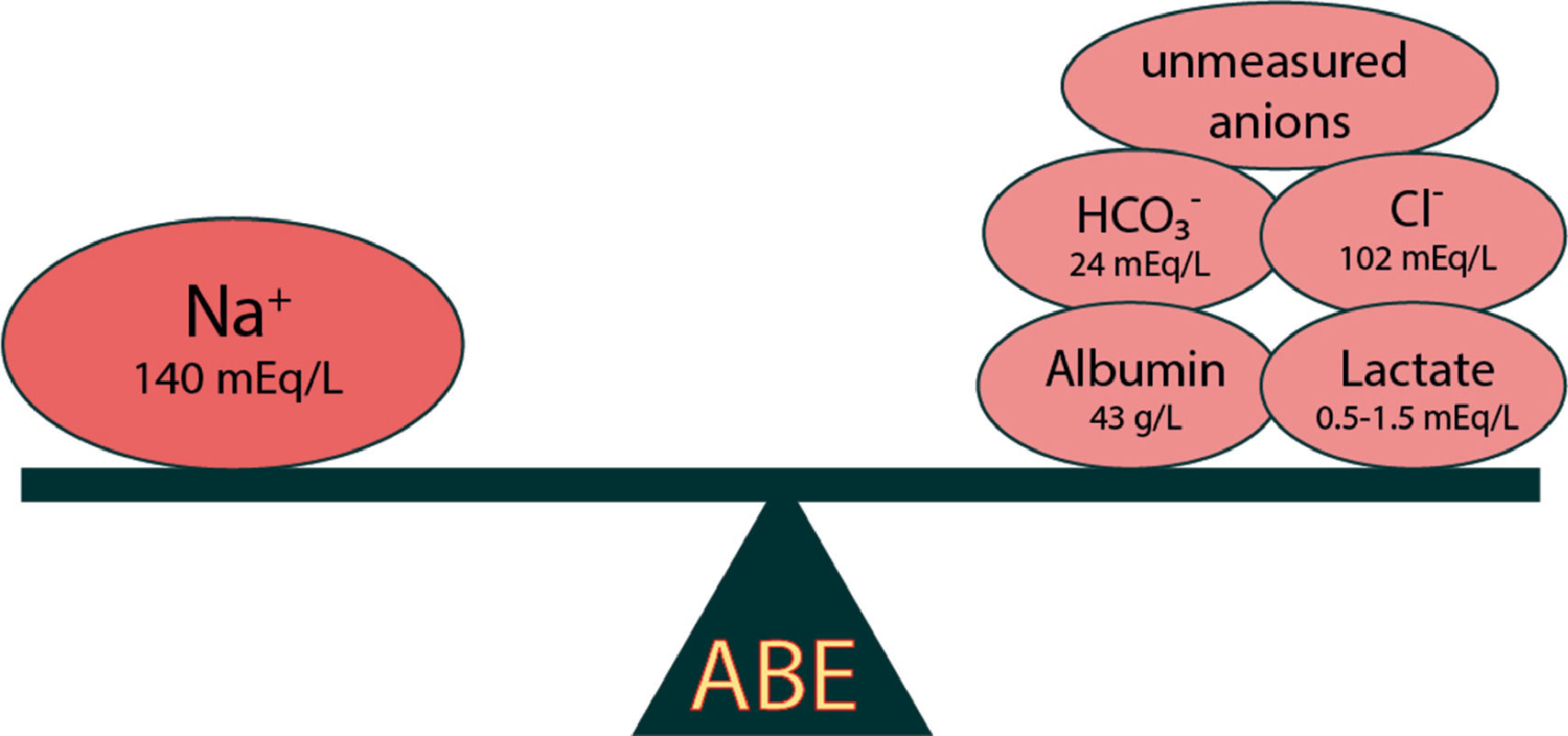
The third physicochemical model was developed by Gilfix in an attempt to interpret and evaluate ABE since Stewart's model is not easily applicable to everyday clinical practice [8]. The model introduces the term "Base Excess" (BE) and includes the following parameters: i) water (H2O) [in particular sodium (Na+) (in dilution and concentration); ii) chloride (Cl−); iii) albumin (Alb); iv) lactic acid (lactate) and v) unmeasured anions (UMA). Positive or negative values of the BE indicator characterise alkalinisation or acidification respectively (Fig. 1) [9].
In more detail,
- i.
Dilution of plasma, due to excess water, causes changes in serum Na+ concentration and leads to Metabolic Acidosis (MAc), in contrast to Metabolic Alkalosis (MAlk) from contraction. The BENa+ is calculated from the equation:
The parameter 0.3, results from the division of SID (normal 40mEq/L) and serum [Na+] which is normally 140mEq/L) (serum [Na+] <133mEq/L is characterized as hyponatraemia).
- ii.
Changes in the concentration of HCO3−, (decrease <22 mEq/L characterizes acidosis while increase >26 mEq/L characterises alkalosis), followed by reverse changes of Cl−, leading to the appearance of hyperchloremic MAc (serum Cl− >109 mEq/L) and hypochloremic MAlk respectively. The BECl− is calculated from the equation:
- iii.
Albumin belongs to the category of non-volatile acids. Thus, hypoalbuminemia indicates loss of acid (weak acid) which results in the appearance of hypoalbuminemia MAlk. The BEAlb is calculated from the equation:
BE albumin >5 mmol/l is characterized as hypoalbulinemic alkalosis
- iv.
Increased lactic acid production and consequently hyperlactemia leads to the appearance of Lactic MAc. The BELactate is calculated from the equation:
- v.
A change in BE that is not due to changes in H2O, Na+, Cl−, Alb Lactate, is characterized by changes in UMA (e.g. ketones, organic acids). The BEUMA is calculated from the equation:
The elements calcium, magnesium, potassium and phosphorus do not seem to play a significant role in ABE as their concentrations are relatively very small compared to those of sodium and chloride [10]. A BEUMA value of < or =-5mEq/L is defined as a clinically significant presence of unmeasured anions.
1.2Liver and ABEThe liver is involved in the regulation of ABE through four pathophysiological mechanisms, a) lactic acid metabolism; b) albumin homeostasis; c) ketogenesis and d) urea production
In more detail,
- a)
The liver is the main site in the metabolism of lactic acid produced per day (70%) [11]. Lactic acid in the liver is firstly metabolized to pyruvic acid and then converted to glucose by gluconeogenesis. Both the release of lactic acid from the muscles and its metabolism into glucose is called the Cori cycle. This complete process results in the equivalent release of an [HCO3−] radical [12]. Lactic acidosis, which is commonly seen in patients admitted to Intensive Care Units (ICU), is a result of reduced lactic acid degradation from the site of production, due to tissue hypoxia (reduced perfusion). The latter is caused by vasoconstriction which is due to the stimulation of the sympathetic-adrenergic axis. Thus, the non-degradation of lactic acid by the muscles, its non-transfer to the liver, reduced metabolism, and the non-production of bases leads to the appearance of lactic acidosis.
- b)
Under normal conditions, albumin is considered to belong to the weak acids. Hypoalbuminemia, either from reduced production (liver failure) or from increased loss (nephrotic syndrome), results in mild MAlk, while hyperalbuminemia, which occurs mainly in dehydrated conditions, is accompanied by mild MAc [13].
- c)
Oxidation of fats in the liver (mitochondria), leads to the production of keto acids (3-hydroxybutyric and acetoacetic acid) that are broken down into ions of H+, which then are excreted by the kidneys. The production and excretion of keto acids is regulated by a reciprocating mechanism. Thus, a decrease of the pH (acidic environment) leads to a decrease in the production of keto acids [14]. On the contrary, increasing the pH increases the production of keto acids [15]. The involvement of hepatic ketogenesis, under normal conditions, in the regulation of ABE is minimal. However, in situations of starvation or alcoholism, the body's attempt to produce energy through fat metabolism, leads to the appearance of a severe degree of MAc.
- d)
In a diet of 100g of protein per day, the produced NH4 (weak neurotoxic acid) amounts to 1mol (1,000mmol). In the liver, NH4 is metabolized to urea, which is excreted by the kidneys. The conversion of NH4 requires the consumption of an equivalent amount of strong base [HCO3−] [16]. Therefore, the production of urea, as an acidification process, plays an important role in the regulation of ABE [17,18].
From what has been mentioned, it appears that the liver is actively involved in the regulation of ABE. Therefore, diseases of the liver, (cirrhosis, ICU patients with cirrhosis, acute or chronic liver failure with or without cirrhosis), result in ABE disorders in one way or another [19]. Furthermore, damage to other organs or systems of the body due to hepatic impairment (kidney failure and hepatic encephalopathy) can exacerbate the already established disorders of ABE [20]. Studies using the usual techniques for approaching ABE disorders in liver disease could not reveal either metabolic or respiratory ABE disorders [10,21]. In contrast, studies using physicochemical models have shown significant ABE disorders in liver disease [5,22]. Following, we will briefly refer to the acidifying and alkalinizing factors which are involved in ABE in liver diseases and in particular in liver cirrhosis.
- 1)
Alkalinizing factors
- a)
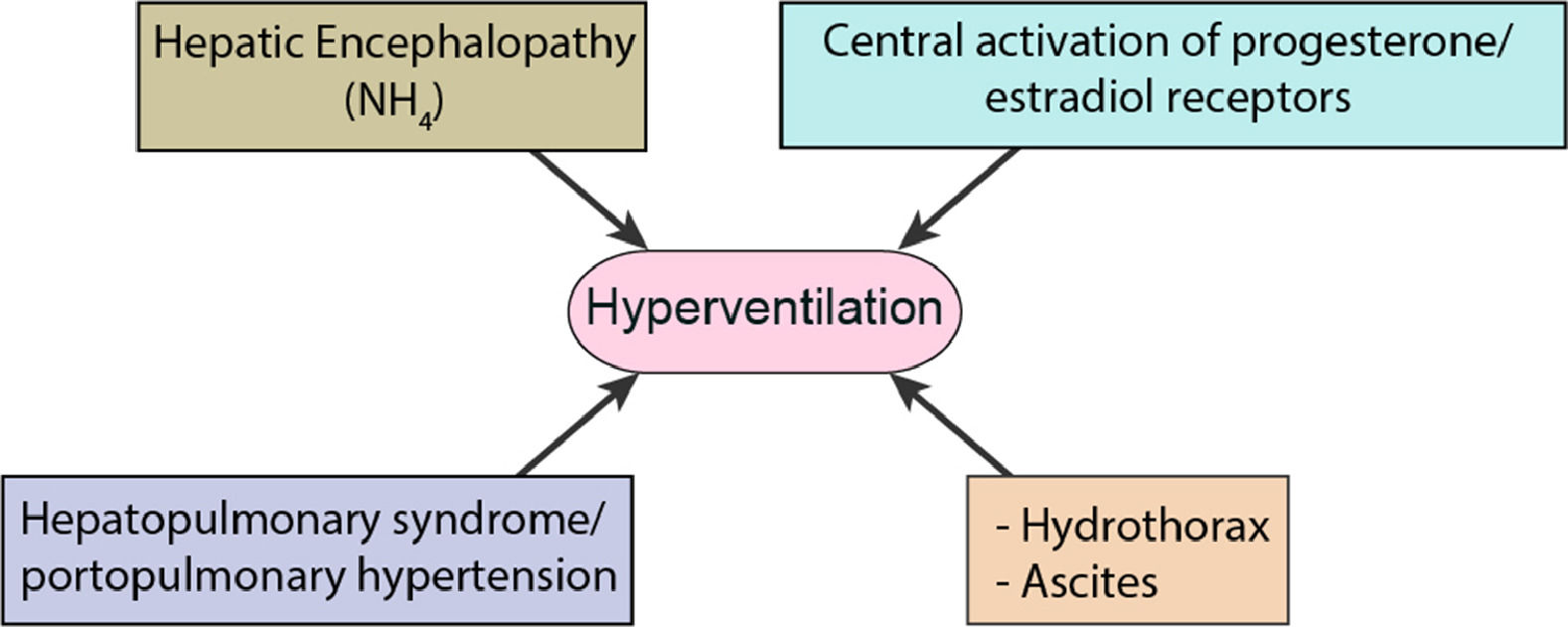
The disorder of ABE in liver diseases which has been identified by both the usual technical and the physicochemical models is Respiratory Alkalosis (RAlk) with marked hypocapnia (Fig. 2) [9,21,23–25]. Growing ascites to a significant degree in combination with hepatic hydrothorax initially causes shortness of breath and hypoxia [26]. Attempting to compensate the body leads to shortness of breath and hyperventilation in the remaining pulmonary parenchyma resulting in hypocapnia. A 55-year-old cirrhotic patient presented by Scheiner at al., appeared to have alkalemic pH with hypocapnia, an indicator of respiratory alkalosis where encephalopathy, ascites and dyspnoea were considered as main factors [9]. Both the increase in the concentration of NH4 and the accompanying development of hepatic encephalopathy, contribute to the hyperventilation of the lungs in the aforementioned pathological condition [27]. A Lustik study has shown that increased concentrations of progesterone and estradiol (reduced hepatic catabolism) may stimulate hyperventilation by stimulating progesterone receptors in the Central Nervous System (CNS) [28].
- b)
The reduced production of urea implies the reduced consumption of [HCO3−] and therefore the appearance of MAlk [29,30]. However, MAlk is not observed in these patients unless diuretics, antacids are administered, if secondary hyperaldosteronism has developed or they have severe hypokalaemia [23,31].
- c)
Hypoalbuminemia is perhaps the most important factor in the development of hypoalbuminemic MAlk in patients with liver cirrhosis. For each decrease of albumin by 1g/dl there is an increase of bases (HCO3−) by 3.7mEq/L [8,32]. It should be noted that the reduction of albumin begins in the early stages of liver cirrhosis due to the reduced intake of protein through food which alters the metabolism of proteins and amino acids.[33] It should also be noted that hypoalbuminemia is the main cause of MAlk in ICU patients [10,34].
- a)
- 2)
Acidifying factors
- a)
Hyponatremia (serum Na+<135mEq/L) is a common (>50%) electrolyte disorder in patients with cirrhosis and ascites [35,36]. Hyponatraemia results from increased reabsorption of H2O by the kidneys (as it is observed in hepatorenal syndrome), through the stimulation of the Renin-Angiotensin system as well as the stimulation of the antidiuretic hormone, due to the reduction of effective circulating blood volume [34,35]. In addition to the increased reabsorption of H2O, repeated therapeutic punctures of ascites play an important role [37]. Dilution hyponatraemia [free water ion H2O retention (pH=7.00), as occurs in patients with liver cirrhosis and ascites], acts as an acidifying factor and consequently leads to acidosis, known as hyponatraemic acidosis [10,27].
- b)
The replacement of HCO3− by Cl− (equilibrium of electric potentials) leads to the appearance of hyperchloramic MAc, another acidifying factor observed in patients with liver cirrhosis and ascites [38,39]. Scheiner et al. present a case of a 46-year-old cirrhotic patient with negative BE and acidaemic pH, indicating metabolic acidosis and after chloride-rich infusions, a hyperchloramic acidosis was observed [9]. In stabilized patients the hyperchloremic MAc, compensates for the main disorder of ABE, which is RAlk. In acute RAlk, the counteraction is almost immediate (5-10min) with the protein and phosphorus (Pi) regulatory systems playing an important role [22,40]. In contrast, in chronic RAlk the compensation (which takes 2-3 days to start acting), is done through the kidneys through two mechanisms: i) the reduced excretion of acids (reduced tubular excretion of H+) and ii) increased tubular excretion HCO3− with correspondingly increased resorption of Cl− (negativeBECl−) [40,41].
- c)
Another cause of hyperchloremic MAc in patients with cirrhosis is the diarrhoea (especially in those taking lactulose) accompanied with loss of HCO3− and Cl− retention, particularly those with hepatic encephalopathy [42]. In addition, these patients show type I renal tubular acidosis (inability to acidify urine, pH>5.3) in a systemic acidosis state [38,43]. This is likely due to reduced outflow which is accompanied by a reduced release of Na+ in the distal tubule and an inability to excrete H+ and Cl− ions in the corresponding section of the renal tubule [9,44].
- a)
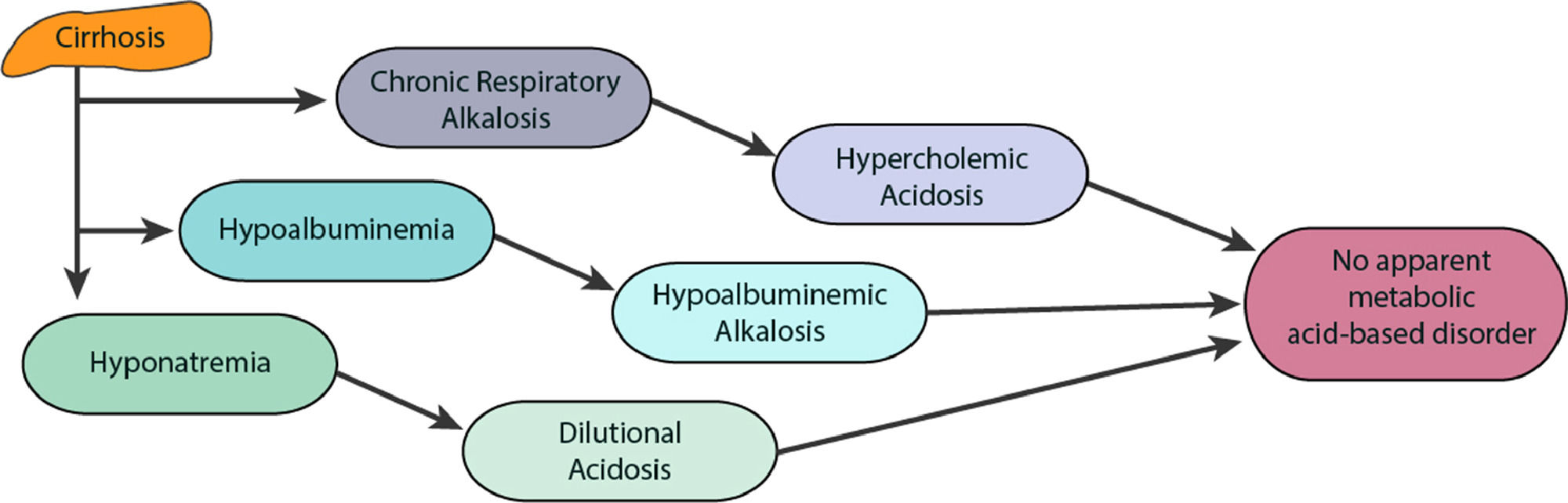
ABE disorders in patients with liver cirrhosis are not fully understood. This is because these disorders involve more than one mechanism that causes both primary and compensatory disorders of ABE. The most common disorder of ABE in cirrhosis is hypocapnic RAlk. However, other disorders develop due to liver dysfunction with mechanisms mentioned above, such as MAc (from dilution and hyperchloremia) and MAlk (from hypoalbuminemia and decreased urea synthesis). The result of these disorders (opposites) of ABE (MAc and MAlk) is that, they are balanced and that patients in stable clinical condition with cirrhosis do not show a change in pH (20%) or where change does occur it is classed as negligible and seldom evaluated [10,45]. This is considered as there is no disruption of a simple ABE disorder, although in this case there is a triple ABE presentation (Fig. 3) [10].
As for hypocapnic RAlk, it is largely compensated by the inadequate excretion of HCO3− from the kidneys, despite their increased reabsorption due to the coexisting hypovolemia (reduction of the effective circulating blood volume). It is worth noting that hepatopulmonary syndrome, which is characterized by the triad, liver disease - dilation of the pulmonary vessels - reduction of blood oxygenation, also contributes significantly to the disorders of ABE in patients with liver cirrhosis [46]. These patients develop intrapulmonary anastomoses (shunts or fistulas) with the diversion of arterial blood to the venous network (right to left) which eventually leads to pulmonary hypertension with consequent changes in blood gases (O2, CO2) [47].
Depending on the clinical picture and which pathological condition prevails over the others, these patients may show: RAlk 44.83%, MAlk 14.28%, MAc 6.12%, RAc 6.12%, MAc + MAlk 8.16%, and normal pH 20.7% [45].
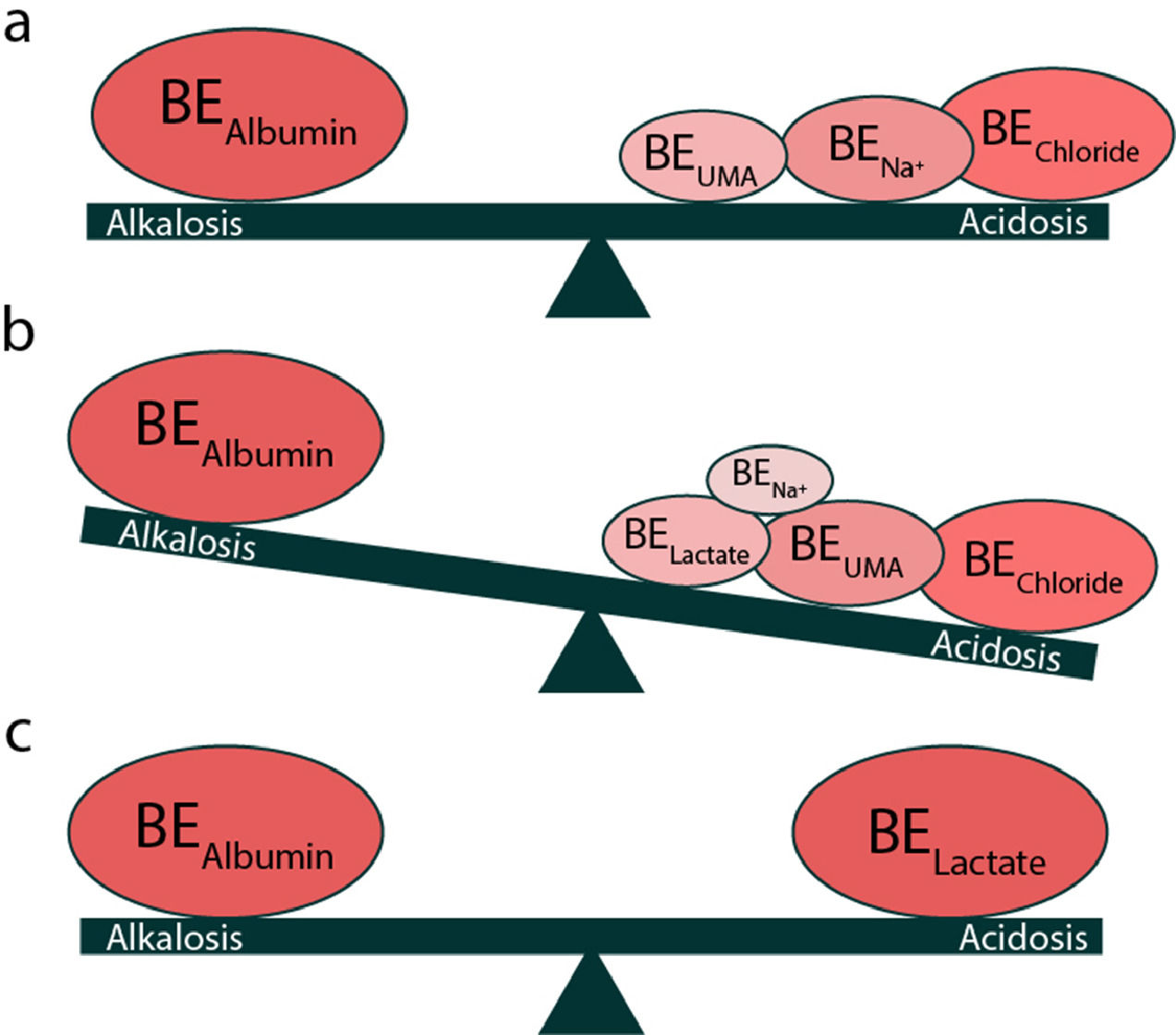
1.4.2ICU patients with cirrhosisAs mentioned previously, in liver cirrhosis, MAlk (due to hypoalbuminemia) coexists with MAc (due to dilution and hyperchloremia) and mainly with RAlk (due to hypocapnia), as well as other ABE disorders, which are/is compensated with the result that the pH remains relatively unchanged [48]. However, in patients with liver cirrhosis who are admitted to the ICU, the prominent disorder of ABE is lactic acidity (>1.9-2.0mmol/L) [49,50]. This increase in lactic acid, on the one hand, is due to increased production (>1500mmol/day) (tissue hypotension-hypoxia, suppression of cellular metabolism due to sepsis, hypercatabolic syndrome) and on the other hand, reduced breakdown in the liver (liver function loss, sepsis) [50–52]. In these patients total BE remains reduced with a predominance of MAc due to an increase in lactic acid, an increase in UMA in contrast to stabilized patients with liver cirrhosis (Fig. 4) [9,10]. These patients have increased mortality due to complications in the function of other organs (multiorgan failure). It should be noted that a small percentage of lactic acid is metabolized within the kidneys (5%) and therefore, in the occurrence of lactic acidosis, participates in the pathology of renal dysfunction that coexists in these patients (hepato-renal syndrome) [53,54].
1.4.3Acute hepatic impairment (acute chronic)Acute liver failure can be caused by extensive burns, acute respiratory failure, and sepsis [55–57]. In these cases, there is an excessive increase in lactic acid production mainly from the visceral areas (and from the lungs in the absence of lung damage) in the context of acute liver dysfunction [58–60].This condition is characterized by stress hyperlactemia (mass glycolysis induced by catecholamines and other cytokines that promote cellular glucose uptake without hypoxia) [61,62]. In milder stages of acute liver damage (I, II) no substantial pH disturbance is observed since the involved mechanisms (compensatory and non-compensatory) are balanced, while in more severe stages (III, IV) lactic acidosis predominates with coexisting RAlk [63].
2ConclusionPatients with liver cirrhosis have ABE disorders such as Respiratory Alkalosis (most common), Metabolic Alkalosis, Metabolic Acidosis, Respiratory Acidosis and mixed disorders (Metabolic Acidosis and Respiratory Alkalosis). The classic reading models of these disorders are not sufficient for clinical recognition. Physicochemical models are often used to calculate the balance between acids and bases in the body for a specific pathological condition. This is due to the pathophysiological mechanisms of cirrhosis, and ABE being mutually reversible, resulting in a significant number of patients with no observable change in arterial blood pH. In these cases, failure to assess the underlying ABE disorders often results in the inadequate and incorrect treatment of the patient's condition.
DeclarationsFunding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials: All data generated or analysed during this study are included in this published article.
Authors' contributions: PK and KPK have equally contributed to the manuscript preparation and review; EP has reviewed the manuscript.
Ethics approval and consent to participate: Not applicable.
Patient consent for publication: Not applicable.
Not applicable.