AKI is known to be associated with increased risk of mortality, however limited information is available on how AKI impacts healthcare costs and resource utilization in hospitalized patients with cirrhosis. Previous studies have had variable definitions of AKI, resulting in inconsistent reporting of the true impact of AKI in patients with cirrhosis.
MethodsData from the Nationwide Inpatient Sample (NIS) which contains data from 44 states and 4378 hospitals, accounting for over 7 million discharges were analyzed. The inclusion data were all discharges in the 2012 NIS dataset with a discharge diagnosis of cirrhosis.
ResultsA total of 32,605 patients were included in the analysis, incidence of AKI was 12.12% in patients with cirrhosis. Crude mortality was much higher for patients with cirrhosis and AKI (14.9% vs. 1.8%, OR 9.42, p<0.001) than for patients without AKI. In addition, mean LOS was longer (8.5 vs. 4.3 days, p<0.001) and median total hospital charges were higher for patients with AKI ($43,939 vs. $22,270, p<0.001). In multivariate logistic regression, controlling for covariates and mortality risk score, sepsis, ascites and SBP were predictors of AKI.
ConclusionsAKI is relatively common in hospitalized patients with cirrhosis. Presence of AKI results in significantly higher inpatient mortality as well as LOS and resource utilization. Median hospitalization cost was twice as high in AKI patients. Early identification of patients at high risk for AKI should be implemented to reduce mortality and contain costs. Prognosis could be enhanced by utilizing biomarkers which could rapidly detect AKI.
Acute kidney injury (AKI) is one of the most severe complications of cirrhosis and portends an ominous prognosis [1]. AKI occurs in approximately in approximately 20% of patients hospitalized with cirrhosis [2]. Two-thirds of these AKI episodes are functional in nature and related to hemodynamic changes in cirrhosis, consisting of splanchnic and systematic arterial vasodilatation with resultant reduction in effective arterial blood volume, whereas remainder of AKI episodes are related to renal structural damage [2,3]. The most common precipitant of renal failure in patients with cirrhosis is bacterial infection [4,5]. Renal failure is considered the most significant independent predictor of death in patients with cirrhosis and spontaneous bacterial peritonitis (SBP) [6]. The prognosis for patients with cirrhosis and renal failure is poor and ICU mortality has been reported as high as 80% [7,8].
Cirrhosis incurs a significant healthcare expenditure burden in the US estimated at US $4 billion and there are limited data on healthcare utilization for the care of patients in AKI with cirrhosis [9]. Chertow et al. addressed the total costs attributed to AKI to be 5% of the overall hospital costs considering all admissions and the estimated annual health care expenditure that were attributable to hospital acquired AKI would exceed $10 billion [10]. Fischer et al. found that dialysis was the most prominent independent factor and was associated with a 62.6% increase in the direct hospital costs [11].
Previous studies have had variable definitions of AKI, resulting in inconsistent reporting of the true impact of AKI in patients with cirrhosis. The majority of previous studies have focused on patients admitted to the intensive care unit, and are thus not generalizable to all hospitalized patients. Currently limited information is available on how AKI impacts healthcare costs and resource utilization in hospitalized patients with cirrhosis. In this study, we attempt to determine the prevalence of AKI in hospitalized patients with cirrhosis using large population administrative database. Furthermore, we examine how AKI effects mortality, hospital length of stay (LOS) and inpatient charges.
2Materials and methods2.1Data collectionPatients were selected from the Healthcare Cost and Utilization Project National Inpatient Sample (NIS) database. This database was created and is maintained by the Agency for Healthcare Research and Quality (AHRQ). It is the largest publicly available all-payer inpatient database in the United States. In the 2012 NIS redesign, a sample of discharges was drawn from all hospitals in the hospital frame. The new systematic sample is a self-weighted sample design similar to simple random sampling. It ensures that the sample is representative of the population on the following critical factors: (1) hospital – unidentified, (2) census division of hospital, (3) hospital ownership, (4) urban–rural location of hospital, (5) hospital teaching status, (6) number of beds in the hospital, (7) diagnosis-related group (DRG) for the hospital stay and (8) admission month of the hospital stay. The 2012 NIS sampling frame covers more than 95 percent of the U.S. population; it includes more than 94 percent of the discharges from U.S. community hospitals.
The NIS collects data from a 20% systematically stratified discharge sample of US hospitals from 44 states and has been reliably used to estimate disease burden and outcomes. Each individual hospitalization is de-identified and maintained in the NIS as a unique entry. Each entry carries information on demographic details including age, sex, race, location of patient's residence, median household income for patient's ZIP Code, payer information, ICD-9-CM diagnosis (up to 25), ICD-9-CM procedures, principal and secondary (up to 15), hospitalization outcome, total charges, length of stay.
2.2Inclusion criteriaThis is a retrospective cohort study of adult patients hospitalized with cirrhosis at acute care hospitals across the United States in 2012. The clinical modifications of International Classification of Diseases, 9th version (ICD-9 CM) diagnostic codes were used to identify the study population. All patients with primary or secondary discharge diagnoses of interest were identified with the following ICD-9 codes: alcohol-induced cirrhosis [571.2], cirrhosis without mention of alcohol [571.5], biliary cirrhosis [571.6]. Study patients with AKI were identified by the primary or secondary discharge diagnosis code(s) of cirrhosis listed above combined with the AKI diagnosis code [584.4–584.9]. The incidence of AKI may have occurred at any point in time during the hospitalization. All patients above 18 years of age were included.
2.3Exclusion criteriaAll patients less than 18 years of age, on dialysis, and without a diagnosis of cirrhosis were excluded from our study cohort.
2.4Outcomes and predictors definitionsPrimary outcomes were incidence of AKI, inpatient mortality, LOS, and hospital charges in cirrhosis related hospitalization. Secondary outcomes included inpatient mortality, LOS, and hospital charges in cirrhotic patients with AKI. Confounders were defined as patient level predictors which included age, race, gender, insurance payer, income quartile. Commonly occurring conditions in cirrhosis were included in the analysis as potential confounders in the development of AKI. These conditions include: hepatitis C [070.51], [070.54], [070.70], Sepsis [995.91, 995.92, 785.52,], diabetes [250.xx], ascites [789.5], SBP [567.23], variceal bleed [456.0]. Case mix severity was controlled for by using the All Patient Refined-Diagnosis-Related Group (APR-DRG) risk of mortality score. The APR-DRG risk of mortality score has been validated as the most discriminative and predictive mortality risk score for cirrhotic patients in the NIS. Hospital level predictors, include location-teaching and bed size of the hospital.
2.5Statistical analysisData were analyzed using SAS version 9.3 (Cary, NC, USA). Descriptive statistics (means, standard deviation, frequencies) were computed for the demographic characteristics. Chi-square analyses examined the association between AKI and the other patient and hospital characteristics. Odds ratios were calculated. Wilcoxon rank-sum tests examined differences in the length of stay and hospital charges by AKI; t-test compared patient age by AKI. These models also included patient characteristics, hospital characteristics, and patient co-morbidities (diabetes, hepatitis C, ascites and variceal bleed). LOS was included as a covariate in the model for total hospital charges. Logistic regression models examined the relationship between AKI and primary outcome of mortality and included the same covariates.
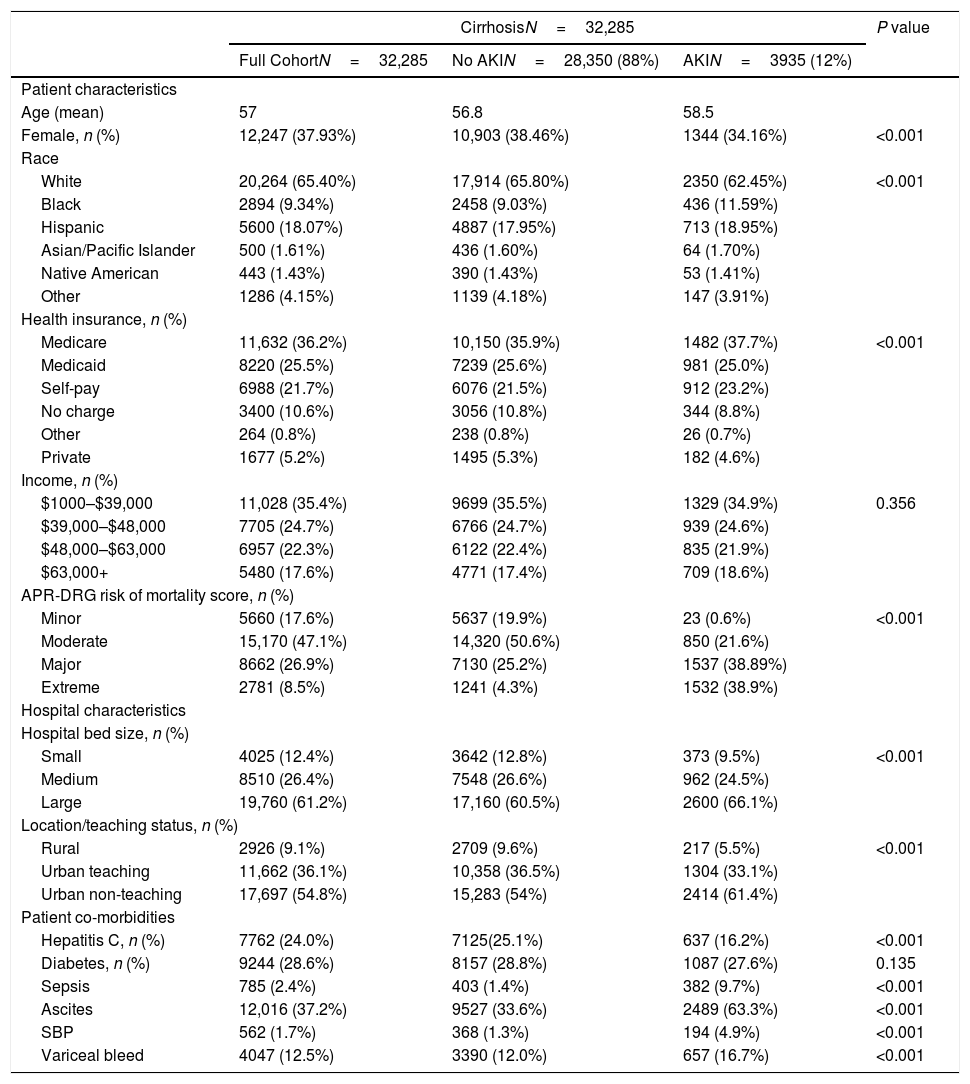
3Results3.1Patient characteristics and univariate analysisIn the 2012 NIS database, we identified 32,285 discharges carrying a primary or secondary diagnosis of cirrhosis (ICD-9 CM: 571.1, 572.2, 571.8). Cirrhotic patients without AKI accounted for 28,350 cases (88%) and with AKI were 3935 (12%) (Table 1). In the full cohort, the mean age was 57 years old, with 38% females. Twenty-four percent carried a diagnosis of hepatitis C, 29% had diabetes, whereas ascites and variceal bleed was seen in 37% and 12% of the patients, respectively. More than sixty percent were covered by medicare or medicaid (36% and 26%, respectively), and 35% of the patients belonged to the lowest income quartile for their zip code. Ninety-one percent of all admissions occurred in urban hospitals, with 36% urban teaching hospitals (Table 1).
Demographics.
| CirrhosisN=32,285 | P value | |||
|---|---|---|---|---|
| Full CohortN=32,285 | No AKIN=28,350 (88%) | AKIN=3935 (12%) | ||
| Patient characteristics | ||||
| Age (mean) | 57 | 56.8 | 58.5 | |
| Female, n (%) | 12,247 (37.93%) | 10,903 (38.46%) | 1344 (34.16%) | <0.001 |
| Race | ||||
| White | 20,264 (65.40%) | 17,914 (65.80%) | 2350 (62.45%) | <0.001 |
| Black | 2894 (9.34%) | 2458 (9.03%) | 436 (11.59%) | |
| Hispanic | 5600 (18.07%) | 4887 (17.95%) | 713 (18.95%) | |
| Asian/Pacific Islander | 500 (1.61%) | 436 (1.60%) | 64 (1.70%) | |
| Native American | 443 (1.43%) | 390 (1.43%) | 53 (1.41%) | |
| Other | 1286 (4.15%) | 1139 (4.18%) | 147 (3.91%) | |
| Health insurance, n (%) | ||||
| Medicare | 11,632 (36.2%) | 10,150 (35.9%) | 1482 (37.7%) | <0.001 |
| Medicaid | 8220 (25.5%) | 7239 (25.6%) | 981 (25.0%) | |
| Self-pay | 6988 (21.7%) | 6076 (21.5%) | 912 (23.2%) | |
| No charge | 3400 (10.6%) | 3056 (10.8%) | 344 (8.8%) | |
| Other | 264 (0.8%) | 238 (0.8%) | 26 (0.7%) | |
| Private | 1677 (5.2%) | 1495 (5.3%) | 182 (4.6%) | |
| Income, n (%) | ||||
| $1000–$39,000 | 11,028 (35.4%) | 9699 (35.5%) | 1329 (34.9%) | 0.356 |
| $39,000–$48,000 | 7705 (24.7%) | 6766 (24.7%) | 939 (24.6%) | |
| $48,000–$63,000 | 6957 (22.3%) | 6122 (22.4%) | 835 (21.9%) | |
| $63,000+ | 5480 (17.6%) | 4771 (17.4%) | 709 (18.6%) | |
| APR-DRG risk of mortality score, n (%) | ||||
| Minor | 5660 (17.6%) | 5637 (19.9%) | 23 (0.6%) | <0.001 |
| Moderate | 15,170 (47.1%) | 14,320 (50.6%) | 850 (21.6%) | |
| Major | 8662 (26.9%) | 7130 (25.2%) | 1537 (38.89%) | |
| Extreme | 2781 (8.5%) | 1241 (4.3%) | 1532 (38.9%) | |
| Hospital characteristics | ||||
| Hospital bed size, n (%) | ||||
| Small | 4025 (12.4%) | 3642 (12.8%) | 373 (9.5%) | <0.001 |
| Medium | 8510 (26.4%) | 7548 (26.6%) | 962 (24.5%) | |
| Large | 19,760 (61.2%) | 17,160 (60.5%) | 2600 (66.1%) | |
| Location/teaching status, n (%) | ||||
| Rural | 2926 (9.1%) | 2709 (9.6%) | 217 (5.5%) | <0.001 |
| Urban teaching | 11,662 (36.1%) | 10,358 (36.5%) | 1304 (33.1%) | |
| Urban non-teaching | 17,697 (54.8%) | 15,283 (54%) | 2414 (61.4%) | |
| Patient co-morbidities | ||||
| Hepatitis C, n (%) | 7762 (24.0%) | 7125(25.1%) | 637 (16.2%) | <0.001 |
| Diabetes, n (%) | 9244 (28.6%) | 8157 (28.8%) | 1087 (27.6%) | 0.135 |
| Sepsis | 785 (2.4%) | 403 (1.4%) | 382 (9.7%) | <0.001 |
| Ascites | 12,016 (37.2%) | 9527 (33.6%) | 2489 (63.3%) | <0.001 |
| SBP | 562 (1.7%) | 368 (1.3%) | 194 (4.9%) | <0.001 |
| Variceal bleed | 4047 (12.5%) | 3390 (12.0%) | 657 (16.7%) | <0.001 |
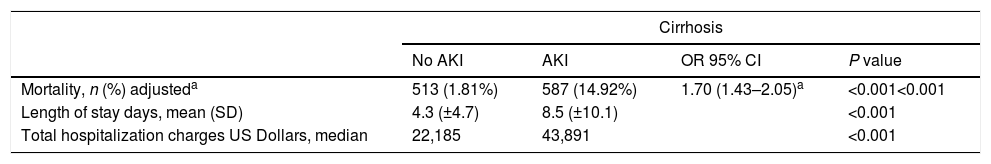
Females were less likely to develop AKI (34% vs 38%, p<0.001) than males. Cirrhotic patients with AKI were more likely to have sepsis (9.7% vs 1.4%, p<0.001), ascites (63% vs 34%, p<0.001), variceal bleed (17% vs 12%, p<0.001), SBP (5% vs 1.7%, p<0.001) but less likely to have hepatitis C (16% vs 25%, p<0.001). There was a significant difference noted for the insurance status, APR-DRG risk mortality score, hospital bed size and hospital location-teaching status between the two groups (p<0.01) (Table 1). There was no difference between these groups with respect to income. The crude in-hospital mortality was significantly higher in the patients with AKI (15% vs 1.8%, p<0.001) compared to the cirrhosis patients without AKI (Table 2). On univariate analysis, progression of AKI was associated with death with an unadjusted odds ratio (OR) of 9.51 (95% CI 8.40–10.77), (p<0.001). After adjusting for confounders patients with AKI had an odds ratio of 1.70 (95% CI 1.43–2.05) (p<0.001) for death, had a longer mean LOS (8.5 days vs. 4.3 days, p<0.001) and higher median total hospitalization charges ($43,939 vs $22,270, p<0.001) (Table 2). As expected, severity of APR-DRG risk of mortality score was associated with a significant increase in mortality, total hospital charges and length of stay in AKI patients compared to patients without AKI (Tables 3 and 4).
Mortality, length of stay, hospital charges.
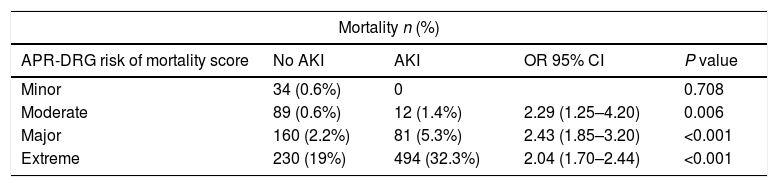
Mortality by APR-DRGa risk of mortality score in cirrhosis with and without AKI.
| Mortality n (%) | ||||
|---|---|---|---|---|
| APR-DRG risk of mortality score | No AKI | AKI | OR 95% CI | P value |
| Minor | 34 (0.6%) | 0 | 0.708 | |
| Moderate | 89 (0.6%) | 12 (1.4%) | 2.29 (1.25–4.20) | 0.006 |
| Major | 160 (2.2%) | 81 (5.3%) | 2.43 (1.85–3.20) | <0.001 |
| Extreme | 230 (19%) | 494 (32.3%) | 2.04 (1.70–2.44) | <0.001 |
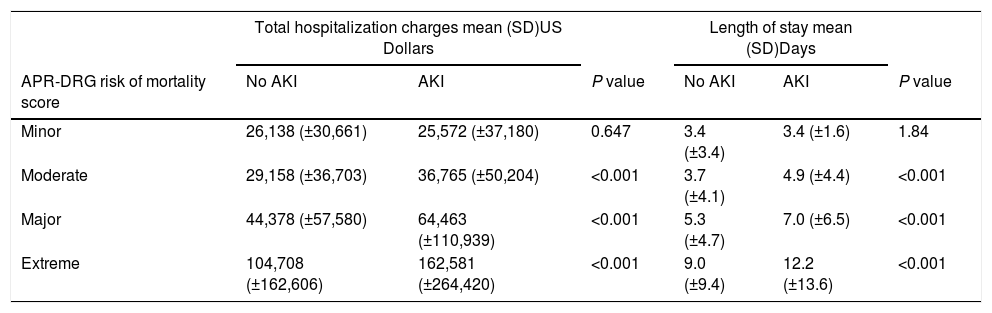
Total charges and LOS by APR-DRGa risk of mortality score in cirrhosis with and without AKI.
| Total hospitalization charges mean (SD)US Dollars | Length of stay mean (SD)Days | |||||
|---|---|---|---|---|---|---|
| APR-DRG risk of mortality score | No AKI | AKI | P value | No AKI | AKI | P value |
| Minor | 26,138 (±30,661) | 25,572 (±37,180) | 0.647 | 3.4 (±3.4) | 3.4 (±1.6) | 1.84 |
| Moderate | 29,158 (±36,703) | 36,765 (±50,204) | <0.001 | 3.7 (±4.1) | 4.9 (±4.4) | <0.001 |
| Major | 44,378 (±57,580) | 64,463 (±110,939) | <0.001 | 5.3 (±4.7) | 7.0 (±6.5) | <0.001 |
| Extreme | 104,708 (±162,606) | 162,581 (±264,420) | <0.001 | 9.0 (±9.4) | 12.2 (±13.6) | <0.001 |
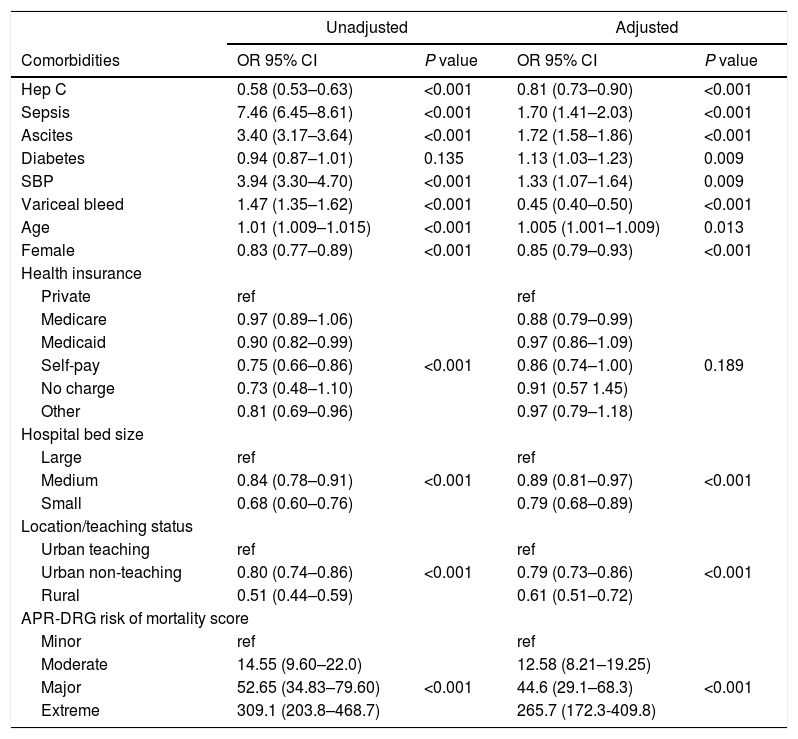
Multivariate regression controlling for covariates and APR-DRG risk score revealed that sepsis was associated with a risk of developing AKI (OR=1.7, 95% CI: 1.43–2.05, p<0.001) (Table 5). Presence of hepatitis C was not found to be associated with AKI. When controlling for APR-DRG mortality score, AKI patients had higher mortality, higher hospital charges, and LOS across the moderate, major and extreme APR-DRG categories (Tables 3 and 4).
Adjusted and unadjusted odds ratios of AKI for cirrhosis related hospitalization.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Comorbidities | OR 95% CI | P value | OR 95% CI | P value |
| Hep C | 0.58 (0.53–0.63) | <0.001 | 0.81 (0.73–0.90) | <0.001 |
| Sepsis | 7.46 (6.45–8.61) | <0.001 | 1.70 (1.41–2.03) | <0.001 |
| Ascites | 3.40 (3.17–3.64) | <0.001 | 1.72 (1.58–1.86) | <0.001 |
| Diabetes | 0.94 (0.87–1.01) | 0.135 | 1.13 (1.03–1.23) | 0.009 |
| SBP | 3.94 (3.30–4.70) | <0.001 | 1.33 (1.07–1.64) | 0.009 |
| Variceal bleed | 1.47 (1.35–1.62) | <0.001 | 0.45 (0.40–0.50) | <0.001 |
| Age | 1.01 (1.009–1.015) | <0.001 | 1.005 (1.001–1.009) | 0.013 |
| Female | 0.83 (0.77–0.89) | <0.001 | 0.85 (0.79–0.93) | <0.001 |
| Health insurance | ||||
| Private | ref | ref | ||
| Medicare | 0.97 (0.89–1.06) | 0.88 (0.79–0.99) | ||
| Medicaid | 0.90 (0.82–0.99) | 0.97 (0.86–1.09) | ||
| Self-pay | 0.75 (0.66–0.86) | <0.001 | 0.86 (0.74–1.00) | 0.189 |
| No charge | 0.73 (0.48–1.10) | 0.91 (0.57 1.45) | ||
| Other | 0.81 (0.69–0.96) | 0.97 (0.79–1.18) | ||
| Hospital bed size | ||||
| Large | ref | ref | ||
| Medium | 0.84 (0.78–0.91) | <0.001 | 0.89 (0.81–0.97) | <0.001 |
| Small | 0.68 (0.60–0.76) | 0.79 (0.68–0.89) | ||
| Location/teaching status | ||||
| Urban teaching | ref | ref | ||
| Urban non-teaching | 0.80 (0.74–0.86) | <0.001 | 0.79 (0.73–0.86) | <0.001 |
| Rural | 0.51 (0.44–0.59) | 0.61 (0.51–0.72) | ||
| APR-DRG risk of mortality score | ||||
| Minor | ref | ref | ||
| Moderate | 14.55 (9.60–22.0) | 12.58 (8.21–19.25) | ||
| Major | 52.65 (34.83–79.60) | <0.001 | 44.6 (29.1–68.3) | <0.001 |
| Extreme | 309.1 (203.8–468.7) | 265.7 (172.3-409.8) | ||
Note: controlled for age, race, gender, health insurance, income quartile, hospital bed size, hospital location/teaching status, APR-DRG risk.
The development of AKI in the setting of cirrhosis has been well recognized to result in a poor prognosis [1]. However, estimates of the incidence of AKI in cirrhosis and attempts to further determine the impact of AKI on mortality have been varying due to a lack of standardization in the definition of AKI. Previous studies have generally examined single center cohorts using creatinine based definition of AKI. The lack of sensitivity inherent in the creatinine based AKI definitions is particularly problematic in patients with cirrhosis where significant muscle atrophy and reduced hepatic conversion of creatinine results in inaccurate creatinine values [12]. Chertow et al. described varying incidence rates of AKI using multiple definitions, ranging from well below 1% for large changes in serum creatinine, 13% for patients with increases in serum creatinine 0.5mg/dl, and 30% when considering smaller but clinically significant changes in serum creatinine [10].
ICD-9-CM codes for acute renal failure had a sensitivity of 35.4%, specificity of 97.7%, positive predictive value of 47.9%, and negative predictive value of 96.1%. As compared with review of medical records, ICD-9-CM codes for ARF had positive predictive value of 94.0% and negative predictive value of 90.0% [13].
For estimates of the incidence of AKI the results of our study show an incidence of 12% compared to 20% as in a previously reported study [2]. The low sensitivity of the code 584.x does provide an underestimation of the actual disease burden when applied to the general population, which likely holds true for our cirrhotic cohort [14]. These discrepancies are also due to differences in case definitions, contrasting clinical with administrative diagnosis of AKI [15].
AKI has been shown to be an independent predictor of death in critically ill patients with liver cirrhosis [1]. The overall mortality in patients with AKI in our study is 15%, which is slighter lower than other AKI studies with heterogeneous critically ill populations admitted to the intensive unit [1,16,17]. Using the acute kidney injury network (AKIN) definition, Belcher et al. found the overall mortality to be 26% in hospitalized patients with cirrhosis with a pronounced stage dependent response with mortality for peak AKIN stages 1, 2 and 3 of 2%, 15% and 44% respectively [12]. A comparable stage dependent response to mortality directly correlating with APR-DRG risk score is seen in our study.
Bacterial infection remains one of the most important challenges in patients with decompensated cirrhosis which often precipitates AKI. The most common bacterial infections are SBP (25%), urinary tract infections (UTI) (20%), pneumonia (15%), and bacteremia (12%) [18]. Follo et al. estimated that approximately one-third of patients with SBP develop renal failure despite the resolution of the infection, which is consistent with our study with 1.7% of the total cirrhotic population developing SBP of which 34% had a diagnosis of AKI at admission or developed it during hospitalization [19]. The high frequency and severity of renal impairment associated with spontaneous bacterial peritonitis (5%) and sepsis (10%) are probably due to the combination of circulatory failure induced by infection and circulatory failure already present as a consequence of cirrhosis [20].
Our findings demonstrate that variceal bleed is also associated with AKI in patients with cirrhosis (12 vs 16.7%), (p<0.001). Several factors related with gastrointestinal bleeding may have a deleterious effect on kidney function. One likely explanation of AKI in this setting is a reduced intravascular volume caused by the blood loss resulting in renal hypoperfusion and higher occurrence of bacterial infections, which develop in the setting of gastrointestinal bleeding [19].
The negative impact of AKI on the natural history of cirrhosis is well described, with the incidence of cirrhotic complications increasing in parallel to the severity of AKI [12]. The development of renal failure is associated with activation of the renin–angiotensin system and a decrease in effective arterial volume. Thus, it has been hypothesized that plasma volume expansion could attenuate the hemodynamic changes in patients with SBP, thereby preserving renal function. A meta-analysis of four randomized trials (with a total of 288 patients) evaluated the impact of albumin infusion (in addition to antibiotics) on renal impairment and mortality in patients with SBP [21]. Albumin infusion was associated with a significant decrease in the incidence of renal impairment (8% vs 31%) and a significant reduction in mortality (16% vs. 35%). Due to the limitations of our database we are unable to account for such interventions in our patient population.
Our findings indicate that the economic burden in AKI is significant and is present across various levels of patient acuity. An important finding of our study is the significant cost associated with AKI in the cirrhosis population. Hospital admissions with chronic liver disease have increased by 21% between 2003 and 2012 with an aggregate cost of $3.3 billion in 2012 [22]. Our results clearly demonstrate, across moderate, major and extreme risk score patients, there is an increase of 26%, 45%, and 55% respectively in total hospital charges from baseline. We believe the incremental cost differential increases as patients are sicker due to the cumulative cost of longer lengths of stays, more prolonged use of expensive therapies, and more likely utilization of intensive care units. Further studies to examine the components of high healthcare expenses in liver disease patients with AKI with be of interest.
There are several limitations to our study. The fidelity of coding to clinical information is imperfect given the nature of administrative database. Healthcare costs are limited to in-hospital charges, indirect and ambulatory charges are not accounted for. Detailed information regarding the sources of the charges cannot be obtained. Definition of AKI was based on discharge diagnosis by hospital coders and not by utilization of lab values or biomarkers using ICA-AKI criteria. Given absence of lab values in our study, specifically creatinine values, ICA-AKI could not be used for patient selection and further similar studies are warranted using the ICA-AKI criteria. MELD score was not able to be calculated, however a marker for degree of illness used was the “All Patient Refined-Diagnosis-Related Group” (APR-DRG) risk of mortality score, which is a validated risk score for disease severity. No information is available regarding treatments and interventions. The limitations of the retrospective nature of our database make it difficult to establish a temporal relationship with infectious processes (i.e. SBP or sepsis) as a precipitating factor for AKI.
In conclusion, cirrhotic patients with AKI incur approximately double the costs and length of stay during their hospitalization compared to non-AKI cirrhotic patients. When controlling for severity of illness, AKI is associated with approximately a two-fold increase in mortality among patients with cirrhosis. Rigorous risk assessment protocols should be implemented to identify those liver disease patients at risk for AKI. Early intervention and treatment should be initiated when AKI is diagnosed given the high mortality and substantial healthcare resource utilization associated with this condition.AbbreviationsAKI acute kidney injury Healthcare Cost and Utilization Project National Inpatient Sample Agency for Healthcare Research and Quality All Patient Refined-Diagnosis Related Group International Classification of Diseases hepatitis C virus length of stay spontaneous bacterial peritonitis acute renal failure acute kidney injury network
None.
Institutional Review BoardThe HCUP-NIS data used in this study represents de-identified, publicly available secondary data, and therefore the study was considered exempt from ethical review.
Author contributionsRaffi Karagozian: Responsible for study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. Gaurav Bhardwaj: Responsible for study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript. Dorothy Wakefield: analysis and interpretation of data; drafting of the manuscript. Elizabeth Verna: critical review of study, study supervision.
Financial disclosuresNone.
Conflict of interestNone.