Drug induced liver injury is a common cause of acute liver failure (ALF). While most of these cases are due to dose dependent hepa-totoxicity with acetaminophen, idiosyncratic drug-induced liver injury (DILI) is responsible for about 15% cases of ALF. Antibiotics are the most common cause of idiosyncratic DILI as well as DILI induced ALF. Etodolac is a selective cycloxygenase- 2 (COX -2) inhibitor non-steroidal anti-inflammatory drug used as an analgesic and anti-inflammatory in musculoskeletal diseases. Severe liver impairment is extremely rare. Till date, only 3 cases of ALF related to etodolac have been reported in the literature. Here we report two cases with a unique presentation of ALF occurring due to DILI caused by etodolac, as diagnosed by Roussel Uclaf Causality Assessment Method (RUCAM).
Drug induced liver injury is a common cause of acute liver failure (ALF). While most of these cases are due to dose dependent hepatotoxicity with acetaminophen, idiosyncratic drug-induced liver injury (DILI) is responsible for about 15% cases of ALF.1 The diagnosis is often of exclusion based on high index of suspicion due to the absence of any clinical, biochemical or histological hallmarks. Antibiotics are the most common cause of idiosyncratic DILI as well as DILI induced ALF. Etodolac a member of pyranocarboxylic acid group is a selective cy-clooxygenase- 2 (COX-2) inhibitor non-steroidal anti-inflammatory drug used as an analgesic and anti-inflammatory in musculoskeletal diseases. Severe liver impairment is extremely rare. Till date, only 3 cases of fulminant hepatic failure due to Etodolac have been reported in the literature.2-4 We report two cases of fulminant hepatic failure occurring due to possible idiosyncratic drug induced liver injury (DILI) caused by Etodolac.
CASE 1A 27-year-old female with no prior history suggestive of liver disease was prescribed etodolac 400 mg and paracetamol 500 mg fixed dose combination twice daily as analgesic for fracture of right forearm following a fall. Her liver function tests before starting etodolac were within normal limits. Within 48 h of drug intake, she developed nausea and vomiting. There was no associated abdominal pain, abdominal distension, constipation, obstipation or fever. Her symptoms gradually worsened and on day three, she became drowsy and icteric.
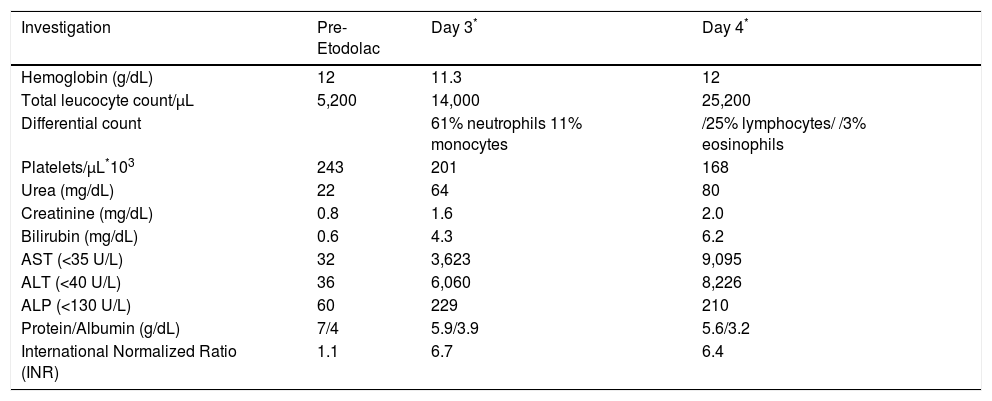
At admission to our institute, the patient was drowsy but responsive, icteric, and had flapping tremors suggestive of grade 2 hepatic encephalopathy (HE, West Haven criteria). The laboratory investigations (Table 1) revealed deranged liver function tests with total bilirubin of 4.3 mg/dL (direct 2.0 mg/dL), aspartate transaminase (AST) of 3,623 U/L, alanine transaminase (ALT) of 6,060 U/L and alkaline phos-phatase (ALP) of 229 U/L. Her International Normalized Ratio was 6.7 and serum creatinine was 1.6 mg/dL (Table 1). Arterial blood gas analysis revealed severe metabolic and lactic acidosis (serum lactate 10.5 mmol/L) with a pH of 6.9 and bicarbonate of 2.9 mEq/L. She also had recurrent episodes of hypoglycaemia and the arterial ammonia levels was 330 μmol/L at admission. The ultrasound showed liver size of 12.4 cm with normal outline and hypoechogenecity. The Doppler of hepatoportal axis was unremarkable. Computerised tomography (CT) of head showed effacement of gyri and sulci with chinking of ventricles suggestive of cerebral edema. Hepatitis B surface antigen, IgM Heptitis B core antibody, anti-hepatitis C virus antibody, serologies for hepatitis A, hepatitis E, cytomegalovirus, ebstein bar virus and herpes simplex virus, and autoimmune markers including antinuclear, antimitochondrial, anti-smooth muscle and anti-liver kidney muscle antibodies were all negative. Serum concentrations of immunoglobulin and ceruloplas-min were also within normal range. The MELD score at admission was 38 and since the patient fulfilled the King's College poor prognostic criteria the family was counselled for an urgent liver transplantation which was declined. The patient was conservatively managed in the Intensive Care Unit with anti-hepatic coma regimen, anti edema measures, N-acetyl cysteine infusion, 25% dextrose infusion, broad spectrum antibiotics, hemodialysis for acute renal failure, and other supportive care. However, her sensorium progressively worsened to grade IV encephalopathy. She rapidly deteriorated and succumbed on the fourth day of her illness due to raised intracranial tension and multiple organ dysfunction.
The laboratory investigations of Case 1.
| Investigation | Pre-Etodolac | Day 3* | Day 4* |
|---|---|---|---|
| Hemoglobin (g/dL) | 12 | 11.3 | 12 |
| Total leucocyte count/μL | 5,200 | 14,000 | 25,200 |
| Differential count | 61% neutrophils 11% monocytes | /25% lymphocytes/ /3% eosinophils | |
| Platelets/μL*103 | 243 | 201 | 168 |
| Urea (mg/dL) | 22 | 64 | 80 |
| Creatinine (mg/dL) | 0.8 | 1.6 | 2.0 |
| Bilirubin (mg/dL) | 0.6 | 4.3 | 6.2 |
| AST (<35 U/L) | 32 | 3,623 | 9,095 |
| ALT (<40 U/L) | 36 | 6,060 | 8,226 |
| ALP (<130 U/L) | 60 | 229 | 210 |
| Protein/Albumin (g/dL) | 7/4 | 5.9/3.9 | 5.6/3.2 |
| International Normalized Ratio (INR) | 1.1 | 6.7 | 6.4 |
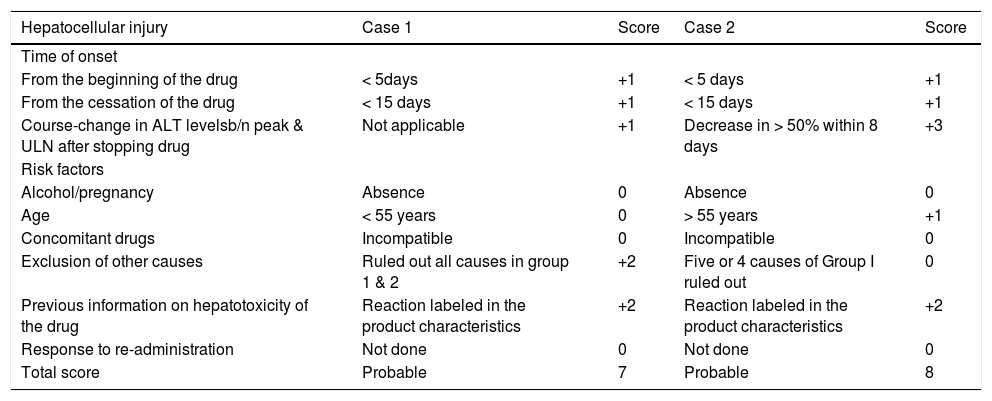
In the absence of other etiologies for ALF, strong history of etodolac intake prior to illness and RUCAM causality instrument score of 7, consistent with probable DILI, we made the diagnosis of Etodolac induced DILI leading to ALF in this case (Table 2).
RUCAM causality assessment score.
| Hepatocellular injury | Case 1 | Score | Case 2 | Score |
|---|---|---|---|---|
| Time of onset | ||||
| From the beginning of the drug | < 5days | +1 | < 5 days | +1 |
| From the cessation of the drug | < 15 days | +1 | < 15 days | +1 |
| Course-change in ALT levelsb/n peak & ULN after stopping drug | Not applicable | +1 | Decrease in > 50% within 8 days | +3 |
| Risk factors | ||||
| Alcohol/pregnancy | Absence | 0 | Absence | 0 |
| Age | < 55 years | 0 | > 55 years | +1 |
| Concomitant drugs | Incompatible | 0 | Incompatible | 0 |
| Exclusion of other causes | Ruled out all causes in group 1 & 2 | +2 | Five or 4 causes of Group I ruled out | 0 |
| Previous information on hepatotoxicity of the drug | Reaction labeled in the product characteristics | +2 | Reaction labeled in the product characteristics | +2 |
| Response to re-administration | Not done | 0 | Not done | 0 |
| Total score | Probable | 7 | Probable | 8 |
An 80-year-old diabetic, hypertensive female with bilateral knee osteoarthritis developed nausea and vomiting after taking 2 doses of etodolac 400 mg and paracetamol 500 mg fixed dose combination as analgesic for knee oste-oarthritis. She became drowsy and icteric on the next day. There was no history of fever, prodromal symptoms, or intake of any new medications other than her usual drugs-amlodipine and metformin-which she regularly took for past few years. The caregivers also denied the use of any herbal or complementary medications. Multiple records of the patient's liver function tests and imaging of liver before admission were within normal limits.
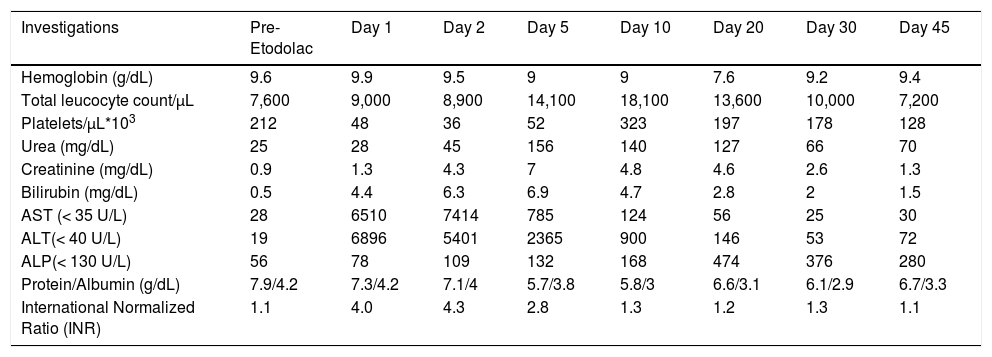
The patient was icteric and drowsy at presentation, flapping tremors (HE Grade 2) could be elicited. The laboratory investigations revealed total bilirubin of 4.4 mg/dL (direct -2.8 mg/dL), AST-6,510 U/L, ALT-6,896 U/L, ALP 78 IU/L, urea 28 mg/dL, creatinine 1.3 mg/dL, and INR 4.0 (Table 3). The arterial ammonia at admission was 300 μmol/L and lactate was 5 mmol/L. The CT head showed signs of cerebral oedema. The ultrasound of liver with Doppler of hepatoportal axis was unremarkable. Etiologi-cal workup done as in case 1 was unremarkable. The MELD score at admission was 31. Over the next two days, her encephalopathy progressed to Grade IV. She became anuric, and serum creatinine rose to 7 mg/dL. She was managed in liver intensive care unit with invasive ventilation, anti-hepatic coma, anti edema measures, N-acetyl cysteine infusion, broad spectrum antibiotics and other supportive care. Intermittent hemodialysis was also done for the acute renal failure. With conservative management her sensorium, liver and renal function, and coagulation paramenters started improving (Table 3). She also developed pneumonia and candidemia during hospital stay which were successfully managed with broad-spectrum antibiotics and antifungals. She was discharged after 45 days of hospitalization and is doing well after 6 months of follow-up with normal liver and renal function test.
The laboratory investigations of Case 2.
| Investigations | Pre-Etodolac | Day 1 | Day 2 | Day 5 | Day 10 | Day 20 | Day 30 | Day 45 |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 9.6 | 9.9 | 9.5 | 9 | 9 | 7.6 | 9.2 | 9.4 |
| Total leucocyte count/μL | 7,600 | 9,000 | 8,900 | 14,100 | 18,100 | 13,600 | 10,000 | 7,200 |
| Platelets/μL*103 | 212 | 48 | 36 | 52 | 323 | 197 | 178 | 128 |
| Urea (mg/dL) | 25 | 28 | 45 | 156 | 140 | 127 | 66 | 70 |
| Creatinine (mg/dL) | 0.9 | 1.3 | 4.3 | 7 | 4.8 | 4.6 | 2.6 | 1.3 |
| Bilirubin (mg/dL) | 0.5 | 4.4 | 6.3 | 6.9 | 4.7 | 2.8 | 2 | 1.5 |
| AST (< 35 U/L) | 28 | 6510 | 7414 | 785 | 124 | 56 | 25 | 30 |
| ALT(< 40 U/L) | 19 | 6896 | 5401 | 2365 | 900 | 146 | 53 | 72 |
| ALP(< 130 U/L) | 56 | 78 | 109 | 132 | 168 | 474 | 376 | 280 |
| Protein/Albumin (g/dL) | 7.9/4.2 | 7.3/4.2 | 7.1/4 | 5.7/3.8 | 5.8/3 | 6.6/3.1 | 6.1/2.9 | 6.7/3.3 |
| International Normalized Ratio (INR) | 1.1 | 4.0 | 4.3 | 2.8 | 1.3 | 1.2 | 1.3 | 1.1 |
Her RUCAM score was 8, consistent with probable DILI, and the diagnosis of DILI due to Etodolac leading to ALF was made (Table 2).
DiscussionIdiosyncratic DILI is responsible for almost 15% of all causes of ALF.5,6 The diagnosis is often one of exclusion, based on a high index of suspicion, and ruling out other causes of liver dysfunction. Antibiotics are the most common cause of DILI as well as DILI induced ALF, followed by herbal supplements, antiepileptics, and nonster-oidal anti-inflammatory drugs.6 A large multicentric prospective study from United States showed that 11.1% cases of ALF were related to idiosyncratic DILI, of which NSAIDS accounted for 5.3% cases.5
Etodolac is a Cox 2 selective NSAID and member of pyranocarboxylic acid group. Severe liver impairment is exceedingly rare, occurring in less than 0.3%.2 Three cases of etodolac induced liver failure have been described in literature.3,5,7 The first case was reported by Mabee CL, et al. in 1995.3 The second case of etodolac induced ALF was described in DILI - ALF study by Reuben A, et al. in 2010.2 Sampaziotis F, et al. described third case of etodolac induced liver failure in 2012.
Both our cases developed ALF after a short duration of exposure to etodolac, had a similar pattern of liver injury, and the one patient who survived showed rapid recovery in liver functions. Both cases met the RUCAM criteria for etodolac induced DILI,8 which has been widely validated to ascertain the presence of DILI. The extreme elevation of transaminases along with hyperbilirubinemia, coagu-lopathy, and cerebral edema within 24-48 h of drug inges-tion argues strongly in favour of a drug induced ALF, which is further bolstered by the fact that liver injury resolved rapidly on cessation of drug in the surviving patient. This pattern was consistent with that of acute hepatic necrosis, which presents with nausea, weakness, fatigue, abdominal pain, mental clouding and multi-organ dysfunction, with a short latency of 1-14 days from the hepatotoxin intake.4 Ischemic hepatitis was a close differential diagnosis, but the presence of severe coagulopathy and cerebral edema within 24-48 h of presentation deemed it unlikely.
ConclusionHepatotoxicity due to etodolac, though rare, may lead to severe hepatic failure, which may present within days of initiation, to months after therapy. The knowledge of this potential adverse effect and fatal consequences warrants immediate discontinuation and close monitoring of patients who develop early symptoms of hepatotoxicity.
Abbreviations- •
ALF: acute liver failure.
- •
ALP: alkaline phosphatase.
- •
ALT: alanine Transaminase.
- •
AST: aspartate Transaminase.
- •
COX -2: cycloxygenase: 2.
- •
CT: computerised tomography.
- •
DILI: drug-induced liver injury.
- •
HE: hepatic encephalopathy.
- •
NSAIDS: non-steroidal anti-inflammatory drugs.
- •
RUCAM: Roussel Uclaf Causality Assessment Method.
None.