Autoimmune hepatitis (AIH) may present acutely, which can rapidly progress to fulminant type. This pattern has been described worldwide but is generally under-reported. We aim to describe the clinical presentation and treatment outcomes of patients with acute onset AIH.
Materials and methodsA multicenter retrospective cohort study of patients with acute onset AIH. Clinical, biochemical, and histological data were analyzed and the outcomes were reported.
ResultsSeventy patients were included. The mean age was 33.8±1.5 years and 58.6% were female. Upon initial presentation, 94% had jaundice, 44% had fatigue, 31% had pruritus, and 29% had abdominal pain. Biochemical analysis revealed elevated alanine transaminase (733±463.6), aspartate transaminase (699±423), and total bilirubin (210±181.8). Antinuclear antibody (ANA) was positive in 61% of patients, anti-smooth muscle antibody (ASMA) in 69%, and both in 31%; immunoglobulin G (IgG) was elevated in 86% of patients. Advanced fibrosis was found in 39%. Complete remission was achieved in 74.3%, two patients required liver transplants and six died. No specific biomarkers were identified as predictive of remission; however, advanced age was associated with poor prognosis.
ConclusionAcute onset AIH is a disease that requires early diagnosis and management. We confirmed that elevated transaminases are the hallmark of biochemical presentation of acute AIH. High IgG, ANA and ASMA are typically present in such patients upon presentation, however, their absence does not totally exclude the diagnosis. Initial response to treatment was excellent; however, the long-term mortality was higher than the general patient population.
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown etiology with variable patterns of presentation. The majority of patients experience chronic insidious onset manifested by an asymptomatic elevation of liver enzymes. However, this disease may present as acute onset hepatitis, fulminant hepatic failure, chronic insidious hepatitis, or well-established cirrhosis. In 20–30% of cases, AIH causes jaundice and a subset of these patients go on to fulminant or subacute liver failure [1–3]. This pattern has not been extensively described in the medical literature and represents a challenge for physicians as it can be very similar to other types of acute hepatitis; furthermore, it may quickly progress to fulminant hepatic failure [1]. Unfortunately, the international AIH group criteria and the simplified criteria have a limited role in scoring this subtype of AIH [4,5]. Some investigators have suggested that acute onset AIH may be a sequence of spontaneous flares of a pre-existing chronic disease [6]. Acute AIH affects all age groups, however, it is more prevalent in children and young adults. Fulminant hepatic failure develops more often in younger age groups [7]. Tokumoto et al. divided acute onset AIH into two distinct types based on the clinical and histological findings: the first is an acute exacerbation of chronic hepatitis and the second is acute onset AIH with clinical and histological features of acute hepatitis without signs of chronicity [8]. Elevated serum aminotransferase levels and the classical histological features are present in all types of AIH [9–11]. The most pressing obstacle hindering effective treatment of acute onset AIH is obtaining an accurate diagnosis and distinguishing this disorder from other acute liver diseases. In this study, we aim to describe the clinical presentation, response to therapy, and outcome of patients with acute onset AIH.
2Materials and methodsThis retrospective, multicenter, cohort study included all adult patients diagnosed with AIH from three tertiary care hospitals in Riyadh, Saudi Arabia between January 1999 and December 2017. As there were no specified guidelines or consensus on the diagnosis of acute onset AIH, diagnosis was based on the revised international AIH group scoring system [4]. Only patients who presented acutely with definite AIH (score>15) or probable AIH (score 11–15) with a favorable response to steroids were included. Acute AIH was defined as the recent onset of hepatitis symptoms (jaundice, fatigue, right upper quadrant pain, and/or anorexia) for less than six months and a minimal 10-fold rise in serum transaminases relative to the upper limit of normal values in someone who had healthy liver function tests for at least six months prior to presentation. Patients with AIH who did not fulfill the acute onset AIH criteria were labeled as classical AIH and were excluded from this analysis. Patients who were known to have other acute or chronic liver diseases including viral hepatitis (A, B, C), metabolic, vascular, or other autoimmune liver diseases (primary biliary cholangitis or primary sclerosing cholangitis) were also excluded. Additional exclusion criteria were exposure to potentially hepatotoxic medication within six months prior to the onset of symptoms and prior organ transplantation.
Demographic data, medical history, laboratory tests including: liver function tests (LFTs): alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase, total bilirubin (TB), and serum albumin; hematological tests: complete blood cell counts, international normalized ratio (INR); immunological tests: antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-mitochondrial antibody (AMA), liver-kidney microsomal antibody, and immunoglobulin G (IgG) were collected upon initial presentation and at every follow-up visit. Histopathological findings, radiological findings, and medications prescribed together with LFTs and other laboratory tests were recorded at follow-up visits. All patients underwent a liver biopsy prior to the initiation of treatment. The grade of inflammation and stage of fibrosis of the liver biopsy were reported according to the METAVIR scoring system [12,13]. The outcome of treatment was reported as complete, partial, or no response. Complete response was defined as the normalization of transaminases within one year of treatment with the disappearance of symptoms, partial response was defined as transaminases improving by at least 50% from baseline tests without complete normalization, and treatment failure was used to indicate no improvement in LFT or that the patient had died or required liver transplantation. Since our aim was to retrospectively analyze the clinical presentation, the biochemical and serological response to therapy and outcomes of patients presented with acute onset AIH, further testing was not attempted. Furthermore, due to the nature of the retrospective trials, it was not possible to calculate a pre-study sample size but rather to include all the cases that met the inclusion criteria.
2.1Ethical considerationsDue to the retrospective nature of this study, patient consent was not required, however, the study was approved by the institutional review boards of the participating hospitals. All procedures were conducted in accordance with the 1975 Declaration of Helsinki.
2.2Statistical analysesA descriptive and inferential analysis was performed using SPSS Package version 17 for Windows (SPSS, Chicago, IL, USA). Categorical data were described as frequency and percentage and compared through the chi-squared or Fisher's exact tests. Numerical data were described as mean and standard deviation and compared by the Student's t-test for normally distributed variables or as median and range and were compared using the Mann–Whitney U test if they were not normally distributed. Prognostic variables predictive of outcome were determined using univariate and multivariate logistic regression analyses. Survival curves were estimated using the Kaplan–Meier method and differences were compared using the log-rank test. A p-value of<0.05 was considered statistically significant.
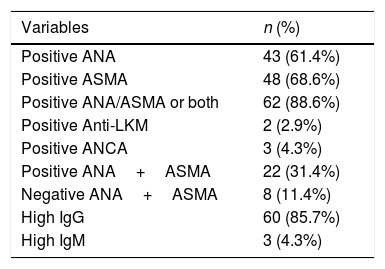
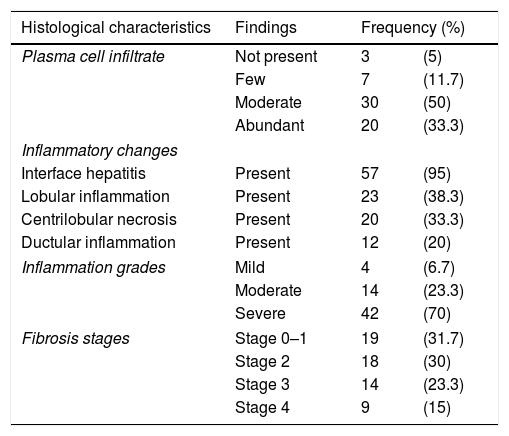
3ResultsDuring the study period, 70 patients were diagnosed with acute onset AIH. The baseline characteristics of these patients are presented in Table 1. The mean age at presentation was 33.8±1.5 years. Females were predominantly affected and the female to male ratio was 1.4:1. The most common symptoms were jaundice (94%) followed by fatigue (44%), itching (31%), and abdominal pain (29%). Diabetes mellitus was found in seven patients (10%) and thyroid disease in five patients (7.1%). Positive ANA, ASMA, and elevated IgG were seen in 61%, 69%, and 86% of patients respectively. The frequencies of positive immunological markers are listed in Table 2. The mean IgG level was 28.3±13.5, and the mean IgM level fell within the normal range. Biochemically, all patients had a significant rise in their transaminases, 50% had elevated INR, and all had normal renal function. Normal TB levels were seen in 5%, ALP in 33%, and albumin in over 40% of the patients with acute onset AIH. Liver biopsies were obtained from all patients prior to commencing treatment, however, the histopathology slides were only available for 60 patients (86%). For the remaining 10 patients, diagnosis was reliant on the available histopathology report, all of which described typical AIH pathology. Detailed histological findings are shown in Table 3. The inflammation was severe (Grade 3) in 70% of the cases and 38.7% had advanced fibrosis upon presentation (Stage 3–4). After a median follow up of 85 months (range: 1–212), complete response and clinical remission were achieved in 52 (74.3%) and incomplete response in 10 (14.3%) patients. Only two patients (2.86%) required liver transplantation and six (8.6%) died during the acute event.
Baseline demographic, clinical and laboratory features of patients presented with acute autoimmune hepatitis.
| Variables | Acute AIH | |
|---|---|---|
| n=70 | % | |
| Demographic features | ||
| Age (Mean±SD) | 33.8±1.5 | |
| Gender | ||
| Female | 41 | 58.6% |
| Male | 29 | 41.4% |
| Clinical presentation | ||
| Jaundice | 66 | 94.3% |
| Fatigue | 30 | 43.5% |
| Pruritus | 22 | 31.4% |
| Abdominal pain | 20 | 28.6% |
| Fever | 12 | 17.1% |
| Hepatomegaly | 6 | 8.6% |
| Splenomegaly | 6 | 8.6% |
| Joint pain | 5 | 7.1% |
| Laboratory factors | ||
| Bilirubin (mmol/L): Mean±SD | 210±181.8 | |
| Albumin (g/L): Mean±SD | 32.5±7.0 | |
| AST (U/L): Mean±SD | 699±423 | |
| ALT (U/L): Mean±SD | 733±463.6 | |
| ALP (U/L): Mean±SD | 258.8±194.6 | |
| GGT (U/L): Mean±SD | 190.3±256.2 | |
| IgG Level (g/L): Mean±SD | 28.3±13.5 | |
| WBC (109/L): Mean±SD | 7.2±3.05 | |
| Hemoglobin (g/L): Mean±SD | 12.7±2.0 | |
| Platelets (109/L): Mean±SD | 272±99.9 | |
| INR (Mean±SD) | 1.6±0.6 | |
AIH: autoimmune hepatitis. SD: standard deviation. AST: aspartate aminotransferase. ALT: alanine aminotransferase. ALP: alkaline phosphatase. GGT: gamma-glutamyl transferase. IgG: immunoglobulin G. INR: international normalized ratio.
Immunology markers of all patients presented with acute onset AIH.
| Variables | n (%) |
|---|---|
| Positive ANA | 43 (61.4%) |
| Positive ASMA | 48 (68.6%) |
| Positive ANA/ASMA or both | 62 (88.6%) |
| Positive Anti-LKM | 2 (2.9%) |
| Positive ANCA | 3 (4.3%) |
| Positive ANA+ASMA | 22 (31.4%) |
| Negative ANA+ASMA | 8 (11.4%) |
| High IgG | 60 (85.7%) |
| High IgM | 3 (4.3%) |
AIH: autoimmune hepatitis. ANA: antinuclear antibody. ASMA: anti-smooth muscle antibody. Anti-LKM: liver/kidney microsomal antibody. ANCA: Antineutrophil cytoplasmic antibodies. IgG: immunoglobulin G. IgM: immunoglobulin M.
Histological characteristics on liver biopsy of 60 patients with acute AIH.
| Histological characteristics | Findings | Frequency (%) | |
|---|---|---|---|
| Plasma cell infiltrate | Not present | 3 | (5) |
| Few | 7 | (11.7) | |
| Moderate | 30 | (50) | |
| Abundant | 20 | (33.3) | |
| Inflammatory changes | |||
| Interface hepatitis | Present | 57 | (95) |
| Lobular inflammation | Present | 23 | (38.3) |
| Centrilobular necrosis | Present | 20 | (33.3) |
| Ductular inflammation | Present | 12 | (20) |
| Inflammation grades | Mild | 4 | (6.7) |
| Moderate | 14 | (23.3) | |
| Severe | 42 | (70) | |
| Fibrosis stages | Stage 0–1 | 19 | (31.7) |
| Stage 2 | 18 | (30) | |
| Stage 3 | 14 | (23.3) | |
| Stage 4 | 9 | (15) | |
From the univariate analysis, no statistically significant variables were found to predict response to treatment in this cohort, however, this could be a type II error due to the small sample size (Table 4). On the other hand, older age at presentation was a predictor of poor outcome on univariate and multivariate analysis (Tables 5 and 6). We studied the rate of disease progression and poor outcome (liver transplant or death) relative to the stage of liver fibrosis and found a slower progression course and a better outcome in patients with mild fibrosis compared to those with severe fibrosis (Fig. 1). All patients received induction with moderate doses of oral prednisolone (30–60mg daily) with gradual weekly tapering and later treatment (usually within 2–4 weeks) with azathioprine (50mg daily, dose adjusted according to response). The two patients who did not achieve complete response were shifted to mycophenolate.
Predictors of response in patients with acute onset AIH by univariate analysis.
| Variables | Remissionn=52 | No remissionn=18 | P value |
|---|---|---|---|
| Age (Mean±SD) | 32.7±1.4 | 37.0±1.8 | 0.28 |
| Gender (male) (%) | 38.5% | 50% | 0.39 |
| Diabetes mellitus (%) | 7.7% | 16.7% | 0.27 |
| Thyroid disease (%) | 9.6% | 0% | 0.17 |
| Total bilirubin: Median (Range) | 137 (14–623) | 204 (14–664) | 0.60 |
| ALT: Median (range) | 573 (400–2368) | 548 (400–1312) | 0.79 |
| ANA (%) | 63.8% | 57.1% | 0.64 |
| ASMA (%) | 63.5% | 83.3% | 0.12 |
| IgG: Median (range) | 24.1 (10.0–74.7) | 31 (15.0–58.1) | 0.25 |
| Inflammation (grade) (1/2/3)a | 15.4/30.7/53.8% | 6.5/21.7/71.8% | 0.63 |
| Fibrosis (stage) (0–2/3–4)a | 53.8/46.2% | 64.4/35.6% | 0.14 |
AIH: autoimmune hepatitis. ALT: alanine aminotransferase. ANA: antinuclear antibody. ASMA: anti-smooth muscle antibody. IgG: immunoglobulin G.
Predictive variables associated with the poor outcome (Death or liver transplant) in acute AIH by univariate analysis.
| Variables | Favorable outcomeN=62 | Poor outcomeN=8 | P value |
|---|---|---|---|
| Age (Mean±SD) | 32.2±1.3 | 46.1±2.0 | 0.01 |
| Gender (male) (%) | 43.5% | 25% | 0.32 |
| Diabetes mellitus (%) | 8.1% | 25% | 0.13 |
| Thyroid disease (%) | 8.1% | 0 | 0.41 |
| Total bilirubin: Median (Range) | 134 (14–664) | 205 (38–461) | 0.47 |
| ALT: Median (range) | 576 (400–2368) | 554 (400–776) | 0.96 |
| INR: Median (range) | 1.4 (1–4.1) | 1.5 (1.1–3.1) | 0.82 |
| ANA | 62.3% | 66.7% | 0.88 |
| ASMA | 66.1% | 87.5% | 0.22 |
| IgG: Median (range) | 23.7 (10–74.7) | 32 (13.0–58.2) | 0.44 |
| Inflammation (1/2/3)a | 9/19.6/71.4% | 12.5/37.5/50% | 0.41 |
| Fibrosis (0–2/3–4)a | 30.6% | 75% | 0.01 |
AIH: autoimmune hepatitis. ALT: alanine aminotransferase. ANA: antinuclear antibody. ASMA: anti-smooth muscle antibody. IgG: immunoglobulin G.
Multivariate analysis of the variables contributing to the prediction of poor outcome (death or liver transplant) in acute onset AIH.
| Variables | OR | 95% CI | P value | |
|---|---|---|---|---|
| Age | 1.109 | 1.011 | 1.216 | 0.03 |
| Gender | 6.309 | 0.215 | 184.836 | 0.29 |
| Diabetes mellitus | 0.763 | 0.040 | 14.412 | 0.86 |
| Total bilirubin | 1.000 | 0.994 | 1.007 | 0.92 |
| ALT | 0.999 | 0.994 | 1.007 | 0.55 |
| INR | 1.784 | 0.449 | 7.092 | 0.41 |
| IgG | 1.010 | 0.920 | 1.109 | 0.83 |
| Advanced fibrosis | 7.988 | 0.630 | 101.292 | 0.11 |
OR: odds ratio. CI: confidence interval. ALT: alanine aminotransferase. INR: international normalized ratio. IgG: immunoglobulin G.
In this study, we analyzed the clinical, biochemical, and histological features together with treatment outcomes and mortality in patients with acute onset AIH. This presentation is unique and differs from classical AIH. In a previous study, we showed that almost one-third of patients with AIH had an acute presentation at the time of diagnosis [14]. This figure was close to the 36.4% reported for western Saudi Arabia [15]. On the other hand, international reports on acute onset AIH have revealed different rates from different studies [9,16–21]. For instance, when the diagnosis of acute presentation was based on biochemical parameters, acute onset AIH ranged from 5.6% to 26% [17,20]. However, when the definition of acute presentation was based on histological features and the presence of centrilobular necrosis (CLN) the rate of acute onset AIH increased to 31.7% and up to 87% in some reports [16–18]. In our cohort, the age of presentation for acute onset AIH was not different from that reported for classical AIH. However, our patients’ mean age is much lower compared to some international studies that reported a mean age of 42–58 years [22–26]. Moreover, acute onset AIH involves a broad range of age groups (13 to over 80 years) [23,24]. The majority of our patients were female, a pattern that is typical for autoimmune diseases, including AIH, and we confirmed that the acute form was not an exception [19,26]. Based on the biochemical and histological parameters, several previous reports on acute onset AIH subdivided the disease into true (genuine) acute onset AIH and acute exacerbation of preexisting chronic AIH [6,9,17–19,26–28]. This is in line with our study, in which 8.6% of the patients had evidence of splenomegaly, which is suggestive of portal hypertension, and the liver biopsies from close to 40% exhibited advanced fibrosis. This finding is not unusual given that AIH is characterized by an insidious course with phases of exacerbation and remission [29]. The original and simplified diagnostic scoring systems for AIH are less accurate in diagnosing acute onset AIH [4,5]. This traditionally represents a diagnostic challenge that leads to delays in diagnosis and, thus, in the initiation of immunosuppression [16,17,28–30]. Such delays in disease management can allow for disease progression into the fulminant form of AIH, which may require immediate liver transplantation [8,16,22,24]. In our cohort, the relatively long follow-up allowed us to consider treatment response and helped identify cases that could have been excluded based on their initial scores. We used transaminases levels elevated over 10 times the normal upper limit as a part of the definition of acute hepatitis. Previous reports have used a similar definition for acute onset AIH with a serum ALT of 5–10 times the normal upper limit [17–21]. The autoimmune profile showed that ANA and ASMA positivity isolated or both together in about 90% of cases. This is similar to reports by Yamamoto et al. and Fujiwara et al. [22,30], yet higher than described by others [9,16–19,26,28]. Elevated serum IgG is an important biochemical indicator used in the diagnosis of AIH [29]. In the present study, IgG levels were higher compared to what we previously reported for classical AIH [14]. While Dohmen et al. reported a similar trend [19], some authors have reported lower serum IgG compared to chronic AIH and normal IgG in about 30–47% of patients [16–18,28,30]. Histological analysis revealed that the majority of our patients had moderate to severe inflammation, however, it was unclear whether this had an impact on overall prognosis.
Fibrosis was highly prevalent in this cohort of patients despite their acute presentation and was associated with poor survival. Similarly, previous studies on acute onset AIH found severe acute inflammation with CLN in patients with acute onset AIH [18,21,25,27]. Several investigators have shown that 55–65% of patients with acute onset AIH displayed evidence of chronicity upon histological examination [19,23,26,28]. The initial overall response to treatment was excellent with a complete response rate of 80%. The response rate for acute onset AIH was shown to be similar to chronic AIH in several reports [14,16,19,24], however, the reported response rates vary from 36 to 100% [20]. Yamamoto et al., found that the use of pulse steroids did not influence the patient outcome compared to a conventional steroid dosing regimen [22]. This observed difference in response rate may arise from differential definitions of acute onset AIH being employed for diagnosis.
Treatment failure may result in progression to the fulminant form of acute onset AIH, which requires urgent liver transplantation and causes mortality. In this study, 2.9% of the patients with acute onset AIH required liver transplantation and this rate is similar to that reported by Yamamoto et al. [22]. In contrast, Yeoman et al. reported a 48% rate of liver transplantation in their cohort; this might be due to their inclusion of patients with severe (fulminant) forms of acute onset AIH [24]. About one-fifth of our patients did not achieve remission. These patients are more likely to progress into fulminant outcome or even mortality [8,16,22,24]. Yamamoto et al. showed that acute severe presentation of AIH, but not acute exacerbation of chronic AIH, was more likely to progress to death during the follow-up [22]. Unfortunately, in AIH, there are no specific and measurable biomarkers that correlate with its pathogenesis, disease progression, relapse, remission, or response to treatment. However, some biomarkers are used as important therapeutic indicators: micro-ribonucleic acids, soluble programmed death-1 (sPD-1), macrophage migration inhibitory factor (MIF), soluble CD163, B-cell activating factor (BAFF), and metabolite patterns in blood. It has been proposed that these markers possess specific characteristics that potentiate their importance as surrogate markers of the inflammatory process, which can be monitored during treatment [31]. Nishikawa et al. reported that serum BAFF and interferon-γ-inducible protein-10 (IP-10) levels are useful for assessing the degree of liver inflammation in AIH [32]. Aarslev et al. studied patients with active AIH, partial response, and complete response to standard therapy. They found that sPD-1 levels were higher in patients with active AIH and in partial responders compared to complete responders [33]. Assis et al. demonstrated that a MIF polymorphism is associated with disease severity in AIH, as indicated by elevated ALT, and requires maintained steroid therapy [34].
The present study has a few limitations. We were unable to test for any of the aforementioned biomarkers because they were not available for daily clinical practice in our centers. Extensive biochemical profiling should be included in future studies to facilitate identification of prognostic biomarkers. The retrospective nature of this study and the lack of a standardized protocol for treatment and follow up also limit our ability to draw causal connections between the data. Future long-term, multicenter, prospective studies are required to better understand the disease and inform its diagnosis, particularly for patients with acute hepatitis with unclear etiology. Advantages of this study include being a multicenter study with a relatively high number of patients, given the uncommon nature of the disease of interest. Moreover, the long duration of the follow-up enabled an unprecedented investigation into the long-term treatment outcomes of acute onset AIH. This study illuminated several clinically relevant aspects of a relatively rare disease with limited investigation worldwide. The findings described here confirm that early diagnosis and timely treatment are critical toward halting the progression to liver failure, reducing the need for liver transplantation, and increasing survival.
5ConclusionAcute onset AIH is a disease that requires early diagnosis and management to ensure a favorable patient outcome. In this study, we described the clinical, biochemical, and histological features of this disease in addition to the long-term response to therapy and survival. Elevated transaminases and IgG with positive ANA and ASMA are the hallmarks of acute onset AIH, however, the absence of these parameters does not totally exclude a positive diagnosis. A large proportion of our patients displayed considerable fibrosis and cirrhosis despite the acute presentation. Overall, we observed an excellent initial response to treatment; however, the long-term mortality was higher than the general patient population, particularly for patients with cirrhosis.
AbbreviationsAIH autoimmune hepatitis liver function tests alanine transaminase aspartate transaminase alkaline phosphatase gamma-glutamyl transferase total bilirubin international normalized ratio antinuclear antibody anti-smooth muscle antibody anti-mitochondrial antibody immunoglobulin G immunoglobulin M
The authors of this manuscript have not received any grant or funding from public or commercial agencies.
Conflicts of interestNo conflicts of interest to disclose.