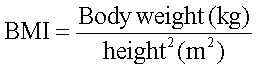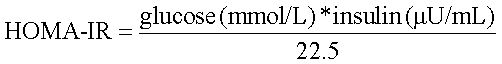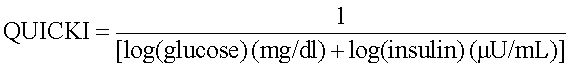Background and rationale. Insulin resistance (IR), adipocytokines, oxidative stress and hepatic apoptosis play a pathogenetic role in nonalcoholic fatty liver disease (NAFLD).
Aims. The evaluation of specific adipocytokines and markers of IR, oxidative stress and apoptosis in NAFLD patients; the introduction of a combined non-invasive index for nonalcoholic steatohepatitis (NASH).
Material and methods. Thirty patients with biopsy-proven NAFLD (15 with simple nonalcoholic fatty liver [NAFL], 15 with NASH) and 24 controls were recruited. Blood samples for total and high molecular weight (HMW) adiponectin, visfatin and tumor necrosis factor (TNF)-α, the apoptotic by-product cytokeratin (CK)–18, the reactive oxygen metabolites (ROMs) and standard biochemical tests were measured. Homeostatic model of assessment - insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated. Main results: Total and HMW adiponectin were significantly lower and TNF-a higher in either NAFL or NASH group compared to control group; CK–18 was significantly higher in NASH compared to either NAFL or control group. CHAI (an acronym of CK–18, HOMA-IR, AST Index) was calculated as the product of parameters being significantly different between NAFL and NASH groups. CHAI was significantly higher in NASH (24.2 [15.1–214.0]) compared to either NAFL (15.7 [6.8–22.7]) or control (5.1 [2.4–7.6]) group (p < 0.001) and significantly higher as the severity of steatosis, fibrosis, ballooning, lobular and portal inflammation advanced.
Conclusion. CHAI was escalating from controls to NAFL and NASH and was higher by increasing the severity of all the main histo-logical lesions. However, a validation study is needed before introducing CHAI in clinical practice.
Nonalcoholic fatty liver disease (NAFLD) is regarded as a global major public health problem, mainly due to the high prevalence of the disease and its association with higher hepatic and cardiovascular morbidity and mortality.1 NAFLD ranges from simple nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), characterized by steatosis, inflammation and fibrosis; advanced NASH may ultimately lead to liver cirrhosis, subacute liver failure and hepato-cellular carcinoma.2
The cross-talk between adipocytokines, insulin resistance (IR) and NAFLD has been reviewed previously.3 Briefly, some adipocytokines may be beneficial, whereas others detrimental for IR and NAFLD pathogenesis. The dynamic balance and interactions between adipocytokines improving or worsening IR lead to the final net result in NAFLD, which may be beneficial or detrimental, respectively.3 The antagonistic interaction between adiponectin and tumor necrosis factor (TNF)-a seem to be a simplified representative example of adipose tissue-liver cross talk.4 However, IR and adipocytokines are not the unique participants in the pathogenesis of NAFLD; hepatic inflammation, apoptosis and oxidative stress are also major interplay contributors.5
The primary endpoints of this study were:
- •
The evaluation of specific adipocytokines, being total and high molecular weight (HMW) adipo-nectin, visfatin (it was initially regarded as an insulin mimetic, but currently known as a proin-flammatory adipocytokine inducing TNF-α, in-terleukin–6 and −1β)6,7 and TNF-α, cytokeratin (CK)-18, which is a by-product of apoptotic process of hepatocytes, and the reactive oxygen metabolites (ROMs) in patients with biopsy-proven NAFLD, as well as their association with the disease severity.
- •
The introduction of a combined non-invasive index for NASH or specific histological lesions. Secondary endpoint was the association of adipo-cytokines and CK-18 with clinical or circulating parameters related to NAFLD.
This was a one-center, cross-sectional study. Patients with NAFLD and controls were recruited on an outpatient basis between June 2008 and November 2010. Determination of eligibility was based on medical history, physical examination, and standard liver function tests (serum aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyl transferase [GGT], alkaline phosphatase [ALP], total and indirect bilirubin) and procedures (liver ultrasound imaging) performed during the screening visit. All participants signed an informed consent. The study protocol was in accordance with the Helsinki Declaration of 1975 and was approved by the local ethics committee. Inclusion criteria for the NAFLD patients were:
- •
Age >18 years.
- •
Bright liver on ultrasound imaging and increased liver function tests for at least 6 months before liver biopsy.
- •
Patient's consent for liver biopsy.
Age- gender- and body mass index (BMI)-matched individuals were recruited for control group. The control group consisted of apparently healthy individuals who underwent regular check-up for professional needs. Inclusion criteria for the controls were:
- •
Age >18 years.
- •
No history of abnormal liver ultrasound imaging or abnormal liver function tests.
- •
Currently normal liver ultrasound imaging.
- •
Currently normal liver function tests.
Controls did not undergo a liver biopsy, because of obvious ethical considerations.
Exclusion criteria for both NAFLD patients and controls were:
- •
Ethanol consumption > 20 g/day.
- •
Liver cirrhosis.
- •
Other liver disease (viral hepatitis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis and overlap syndromes, drug-induced liver disease, hemochromatosis, Wilson's disease, a1-antitrypsin deficiency).
- •
Type I diabetes mellitus.
- •
Pancreatitis.
- •
Uncontrolled hypothyroidism or hyperthyroi-dism.
- •
Adrenal insufficiency.
- •
Renal failure.
- •
Thrombotic disorders.
- •
Cancer.
- •
Pregnancy.
- •
Premature ovarian failure.
- •
Addiction to any drug.
- •
Use of the following medications within a 12-month period before screening: estrogens, progestins, glucocorticosteroids, thiazolidinedio-nes, insulin, sibutramine, orlistat, rimonabant, vitamin E, vitamin C, ursodeoxycholic acid, ferrum, interferon, tamoxifene, amiodarone, biologic agents, folate or vitamin B supplements, antibiotic, any medication against tuberculosis, epilepsy or viruses, or any medication affecting hemosta-sis, such as antiplatelet agents, aspirin or oral anticoagulants.
- •
Use of intravenous glucose administration or parenteral nutrition within a 1-month period before screening.
The patients were initially selected on the basis of their medical history, family history and previously performed laboratory exams, if any. In those initially selected, a set of serological tests was performed at baseline, as follows: HBsAg, anti-HCV, anti-mitochondrial antibody, anti-nuclear antibody, anti-cardiolipin antibody, anti-neutrophil cytoplasmic antibody, anti-smooth muscle antibody, alpha–1 antitrypsin, thyroid stimulating hormone, iron, ferritin, prothrombin time, partial thromboplastin time, platelets count. Furthermore, in specific NAFLD patients, Perl stain, orcein Shikata stain or periodic acid stain were selectively performed on histologic samples, if hemochromatosis, Wilson’s disease or a1-antitrypsin deficiency were suspected.
Morning (8–9 am) fasting serum samples were collected 1–2 h prior to liver biopsy, performed under computed tomography-guidance by an experienced radiologist (EZ) and interpreted by two experienced pathologists (KP, EK). Serum AST, ALT, GGT, ALP, triglycerides, high-density lipoprotein cholesterol (HDL-C), uric acid and glucose were measured within 1 h after blood drawing, with standard methods using an automated analyzer (Olympus AU2700; Olympus, Hamburg, Germany). ROMs were measured within 5 min after blood drawing, with spectrophotometry using an automated analyzer (Free Radical System [FRAS]-3; Diacron International, Grosseto, Italy). This method calculates the total oxidative stress by measuring free oxygen radicals, such as hydroxype-roxides (R-OOH), which are intermediate products of respiratory or oxidative burst; the intra- and inter-assay coefficients of variation (CV) of the method are 2.1 and 3.1%, respectively.
Sera were also immediately frozen at −30 °C for the measurement of insulin, total and HMW adipo-nectin, visfatin, TNF-α and CK-18. Insulin was measured with immuno-chemiluminescence on an Immulite 2500 immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL; intra-assay CV 3.3–5.5%, total CV 4.1–7.3%). Total and HMW adipo-nectin, visfatin, TNF-α and CK-18, were measured with enzyme-linked immunosorbent assay (ELISA) on a ELx800 Absorbance Microplate Reader automated analyzer (BioTek, Winooski, VT, USA), by using the following commercial kits, respectively: adipo-nectin human ELISA kit (Phoenix Europe GmbH, Karlsruhe, Germany; intra-assay CV 5.0%, inter-assay CV 6.0%); adiponectin multimeric ELISA kit (ALPCO Immunoassays, Salem, NH, USA; intra-as-say CV 3.3–5.0%, inter-assay CV 5.7%); visfatin human C-terminal ELISA kit (Phoenix Europe GmbH, Karlsruhe, Germany; intra-assay CV 5.6%, inter-assay CV 7.0%); TNF-α human ELISA kit (R&D Systems, Minneapolis, MN, USA; intra-assay CV 4.2–5.2%, inter-assay CV 4.6–7.4%); M30 Apopto-sence ELISA kit (PEVIVA Bromma, Sweden; intra-assay CV 3.1%, inter-assay CV 5.2%).
BMI was calculated by the formula:
IR was quantified by the homeostatic model of assessment - insulin resistance (HOMA-IR) using the formula:8
Insulin sensitivity was quantified by the quantitative insulin sensitivity check index QUICKI using the formula:9
AST / ALT ratio and HMW/total adiponectin ratio were also calculated.
NAFLD patients were classified in those with NAFL or NASH according to the criteria of NAFLD Activity Score (NAS).10 Patients with NAS < 3 were classified as NAFL, whereas patients with NAS = 3–4 (borderline NASH) or NAS > 4 (definite NASH) or fibrosis stage > 2 as NASH. Steatosis grade, fibro-sis stage, lobular and portal inflammation, and ballooning were categorized based on the classification of NASH Clinical Research Network.10 For the need of this analysis, only NAFLD patients were included, given that the controls did not undergo liver biopsy, as described above. Regarding fibrosis stage, patients with NASH-related cirrhosis (grade 4) were excluded, because:
- •
The hepatic clearance of adipocytokines may be decreased, because of decreased hepatic catabo-lism and decreased effective hepatic blood flow.11
- •
Overwhelming production of proinflammatory cytokines observed in cirrhosis may be independent of obesity and/or insulin resistance, contrary to non-cirrhotic patients with NAFLD.12
Following the between group analysis, we aimed to combine different NAFLD-related parameters in order to introduce a new potential non-invasive marker for NASH or specific histological lesions. According to the post-hoc analysis, parameters being statistically different between NAFL and NASH group were planned to be combined as follows: if a parameter was higher in NASH group, it would serve as a multiplier in the combined index, whereas if a parameter was lower in NASH group, it would serve as denominator.
Statistical analysisContinuous data are presented as median (1st–3rd quartile). Categorical data are presented as numbers. Kolmogorov-Smirnov test was used to check the normality of distributions of continuous variables. Independent samples T-test or Mann-Whitney test were used for between group comparisons, in cases of two groups of continuous variables. Oneway analysis of variance (ANOVA) or Kruskal-Wallis test were used in cases of more than two groups of continuous variables. In case of statistically significant difference in ANOVA or Kruskal-Wallis test, Bonferroni post-hoc adjustment was used for multiple pairwise comparisons. Chi-square test was used to compare categorical variables. Spearman’s coefficient (rs) was used for binary correlations. Receiver operating characteristic (ROC) curve was used and area under the curve was calculated to test whether NASH could be distinguished from NAFL non-invasively. Statistical analysis was performed with SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). Significance was set at p < 0.05.
ResultsThirty patients with biopsy-proven NAFLD (15 with NAFL and 15 with borderline or definite NASH) and 24 controls were included in this series. BMI, waist, AST, ALT, GGT, triglycerides, insulin, HOMA-IR and CK-18 were not normally distributed. Comparative data of the study groups are presented in table 1. There were no statistically significant differences between groups in gender, age, BMI and waist circumference. Between groups comparisons provided a statistically significant trend (ANOVA or Kruskal-Wallis test) in total (p = 0.007) and HMW adiponectin (p = 0.029), TNF-α (p = 0.012) and CK-18 (p = 0.016), but not in visfatin or HMW/total adi-ponectin ratio. In adjusted pairewise comparisons, total and HMW adiponectin were significantly lower and TNF-a higher in either NAFL (p = 0.019, p = 0.043 and p = 0.016, respectively) or NASH (p = 0.031, p = 0.046 and p = 0.014, respectively) group compared to control group; CK-18 was significantly higher in NASH compared to either NAFL (p = 0.037) or control (p = 0.028) group (Table 1). As expected, there was a statistically significant trend towards higher AST (< 0.001), ALT (< 0.001), GGT (< 0.001), ALP (p = 0.015), triglycerides (p = 0.002), uric acid (p = 0.033), glucose (p = 0.031), insulin (p = 0.001) and HOMA-IR (p = 0.001), whereas lower AST/ALT ratio (< 0.001), HDL-C (p = 0.013) and QUICKI (< 0.001) by escalating from control to NAFL and NASH group. There was not statistically significant difference in ROMs (Table 1). Data for specific histological lesions are shown in table 2.
Comparative data of study groups.
| Control | NAFL | NASH | p-value for trend* | Reference range | |
|---|---|---|---|---|---|
| Patients/Women (N) | 24/20 | 15/10 | 15 / 12 | 0.462 | - |
| Age (years) | 56 (52–61) | 55 (44–60) | 54 (50–63) | 0.970 | - |
| BMI (kg/m2) | 30.0 (28.8–31.8) | 30.3 (29.4–36.3) | 34.8 (29.7–39.8) | 0.122 | 20–25 |
| Waist circumference (cm) | 100 (96–102) | 103 (95–109) | 109 (101–113) | 0.070 | Male < 94 Female < 80 |
| AST (U/L) | 19 (17–22) | 24.0 (23–31) | 38 (31–53) †,‡ | < 0.001 | 10–31 |
| ALT (U/L) | 17 (13–23) | 36 (26–52) | 53 (34–82) t | < 0.001 | 10–34 |
| AST/ALT ratio | 1.11 (0.88–1.46) | 0.74 (0.49–1.1) † | 0.86 (0.69–0.93) † | < 0.001 | - |
| GGT (U/L) | 14 (12–19) | 30 (22–53) | 43 (25–101) † | < 0.001 | 0–38 |
| ALP (U/L) | 63 (52–76) | 76 (65–96) † | 79 (50–93) | 0.015 | 30–120 |
| Triglycerides (mg/dL) | 101 (83–133) | 150 (102–165) | 173 (129–261) † | 0.002 | < 150 |
| HDL-C (mg/dL) | 56 (48–68) | 50 (41–57) | 48 (44–52) † | 0.013 | Male > 40 Female > 50 |
| Uric acid (mg/dL) | 4.7 (4.0–5.3) | 5.5 (4.7–6.0) | 5.4 (4.3–6.5) | 0.033 | 2.6–6.6 |
| Glucose (mg/dL) | 88 (80–94) | 90 (83–108) | 102 (92–118) † | 0.031 | 60–100 |
| Insulin (μU/mL) | 4.3 (2.0–6.9) | 7.5 (5.3–12.9) | 12.1 (7.7–24.1) † | 0.001 | 6–27 |
| HOMA-IR | 0.9 (0.5–1.6) | 2.1 (1.1–3.6) | 3.2 (2.0–7.1) †,‡ | 0.001 | na |
| QUICKI | 0.39 (0.36–0.44) | 0.34 (0.32–0.38) † | 0.32 (0.29–0.33) † | <0.001 | na |
| ROMs (UCarr) | 367 (308–424) | 326 (277–360) | 313 (268–384) | 0.550 | 250–300 |
| Total adiponectin (μg/mL) | 8.2 (4.4–11.4) | 3.5 (2.8–7.9) † | 4.0 (3.6–6.3) † | 0.007 | na |
| HMW adiponectin (μg/mL) | 3.5 (2.2–4.9) | 1.9 (1.3–3.4) † | 1.9 (1.6–3.7) † | 0.029 | na |
| HMW/Total adiponectin ratio | 0.43 (0.41–0.47) | 0.48 (0.42–0.53) | 0.46 (0.42–0.53) | 0.255 | na |
| Visfatin (ng/mL) | 6.4 (3.9–7.6) | 5.3 (4.2–6.6) | 5.7 (4.2–7.7) | 0.986 | na |
| TNF-α (pg/mL) | 9.4 (7.1–13.0) | 14.8 (11.6–19.9) † | 15.7 (11.2–20.5) † | 0.012 | |
| CK–18 (U/L) | 250 (215–273) | 257 (215–312) | 310 (220–615) †,‡ | 0.016 | na |
Data are median (1st–3rd quartile) or numbers.
p < 0.05 compared to NAFL group (Bonferroni post-hoc adjustment). ALP: alkaline phosphatase. ALT: alanine transaminase. AST: aspartate transaminase. BMI: body mass index. CK: cytokeratin. GGT: gamma-glutamyl transferase. HDL-C: high density lipoprotein cholesterol. HMW: high molecular weight. HOMA-IR: homeostatic model of assessment insulin resistance. QUICKI: quantitative insulin sensitivity check index. NAFL: nonalcoholic fatty liver (simple steatosis). NASH: nonalcoholic steatohepatitis. ROMs: reactive oxygen metabolites. TNF: tumor necrosis factor.
Data for specific histological lesions (NAFLD patients only; n = 30).
| Histological lesion | Patients (%) |
|---|---|
| Steatosis grade | |
| < 5% | 2 (6.7) |
| 5–33% | 17 (56.6) |
| > 33–66% | 9 (30.0) |
| > 66% | 2 (6.7) |
| Fibrosis stage | |
| None | 10 (33.3) |
| Perisinusoidal or periportal | 14 (46.7) |
| Perisinusoidal and periportal | 5 (16.7) |
| Bridging fibrosis | 1 (3.3) |
| Lobular inflammation | |
| No foci | 19 (63.3) |
| 2–4 foci | 8 (26.7) |
| > 4 foci | 3 (10.0) |
| Portal inflammation | |
| None to minimal | 18 (60.0) |
| Grater than minimal | 22 (40.0) |
| Ballooning | |
| None | 6 (20.0) |
| Few ballon cells | 20 (66.7) |
| Many ballon cells | 4 (13.3) |
Data are numbers (percentage). The classification was based on the criteria of NAFLD Activity Score (NAS).10
By looking into the post-hoc analysis, only AST, HOMA-IR and CK-18 were shown to be significantly different between NAFL and NASH group (Table 1). Based on this observation and the fact that all were higher in NASH group, we calculated the product of these three parameters (divided by 1,000) to possibly multiply their discriminating effect between NAFL and NASH or within histological lesions. We named this product CHAI (an acronym of the words CK-18, HOMA-IR, AST Index). CHAI, which was not normally distributed, was significantly higher in NASH (24.2 [15.1–214.0]) compared to either NAFL (15.7 [6.8–22.7]) or control (5.1 [2.4–7.6]) group (p < 0.001) (Figure 1). In ROC analysis, CHAI could distinguish NASH from NAFL with sensitivity 46% and specificity 80%; the area under the ROC curve was 0.718 (95% Confidence Interval 0.521–0.915).
Subsequently, analysis within specific histologic lesions was performed; in this analysis, only NA-FLD patients were included, given that the controls did not undergo liver biopsy. Within the lesions of steatosis grade, fibrosis stage, ballooning, lobular and portal inflammation, there were not statistically significant differences in total and HMW adiponec-tin, visfatin, TNF-α and ROMs (Table 3). CK-18 was significantly higher in more severe lobular and portal inflammation, but not steatosis, fibrosis or ballooning. Notably, CHAI was significantly higher by increasing the severity of all the five histological lesions (Table 3).
Comparative data of total and HMW adiponectin, visfatin, TNF-α, ROMs, CK-18, and CHAI within specific histological lesions (NAFLD patients only; n = 30).
| Histological lesion | Patients (N) | Total adiponectin (μg/ml) | HMW adiponectin (μg/ml) | Visfatin (μg/ml) | TNF-α (pg/mL) | ROMs (UCarr) | CK-18 (U/L) | CHAI |
|---|---|---|---|---|---|---|---|---|
| Steatosis grade | ||||||||
| (p-value) * | 0.549 | 0.406 | 0.894 | 0.582 | 0.945 | 0.055 | 0.006 | |
| < 33% | 19 | 3.6 (2.8–6.9) | 1.9 (1.3–3.4) | 5.6 (4.2–6.6) | 15.3 (11.9-19.9) | 324 (277-360) | 255 (215-312) | 15.7 (6.7-20.2) |
| > 33% | 11 | 4.1 (3.6-6.3) | 2.6 (1.6-3.7) | 5.7 (3.4-8.5) | 14.7 (9.1-20.5) | 308 (241-384) | 334 (275-615) | 31.1 (18.2-318.2) |
| Fibrosis stage | ||||||||
| (p-value) * | 0.170 | 0.309 | 0.249 | 0.198 | 0.556 | 0.235 | 0.024 | |
| Absent | 10 | 5.2 (3.1-10.2) | 2.6 (1.3-4.2) | 5.2 (4.0-6.0) | 13.8 (11.0-16.6) | 319 (276-410) | 256 (213-315) | 11.5 (6.4-18.0) |
| Present | 20 | 4.0 (3.0-5.2) | 1.8 (1.5-2.7) | 5.7 (4.2-8.3) | 17.1 (12.5-23.1) | 316 (277-358) | 295 (228-483) | 23.5 (14.1-77.0) |
| Lobular inflammation | ||||||||
| (p-value) * | 0.599 | 0.777 | 0.420 | 0.813 | 0.470 | 0.006 | 0.004 | |
| Absent | 19 | 3.9 (3.0-7.9) | 2.1 (1.4-3.4) | 5.6 (4.4-6.6) | 14.9 (11.4-20.1) | 313 (277-360) | 257 (215-312) | 15.9 (7.2-24.2) |
| Present | 11 | 4.0 (3.5-6.2) | 1.7 (1.5-3.7) | 5.7 (3.4-7.7) | 15.6 (11.5-19.7) | 318 (241-547) | 465 (220-1102) | 28.4 (15.1-392.8) |
| Portal inflammation | ||||||||
| (p-value) * | 0.154 | 0.295 | 0.996 | 0.632 | 0.777 | 0.020 | 0.009 | |
| None to minimal | 18 | 4.0 (3.1-8.3) | 2.1 (1.4-3.6) | 5.5 (4.3-6.3) | 14.9 (11.0-20.9) | 327 (303-374) | 268 (213-318) | 15.9 (7.1-23.1) |
| Greater than minimal | 12 | 4.0 (2.8-5.2) | 1.7 (1.5-2.8) | 6.1 (3.5-8.3) | 15.2 (12.5-19.7) | 307 (222-442) | 296 (228-980) | 28.7 (10.3-318.2) |
| Ballooning* | 0.468 | 0.534 | 0.876 | 0.525 | 0.265 | 0.254 | 0.014 | |
| Absent | 6 | 5.7 (3.0-11.0) | 2.7 (1.3-4.1) | 5.5 (4.7-7.2) | 14.3 (9.1-19.0) | 310 (270-368) | 246 (213-289) | 9.9 (5.8-16.1) |
| Present | 24 | 4.0 (3.2-6.1) | 1.8 (1.5-2.8) | 5.7 (3.9-7.5) | 15.7 (11.8-20.1) | 339 (309-410) | 295 (222-436) | 21.1 (13.5-35.6) |
Data are median (1st-3rd quartile) or numbers.
Between groups comparison (independent samples T-test or Mann-Whitney test). AST: aspartate transaminase. CHAI: CK-18, HOMA-IR, AST index. HMW: high molecular weight. CK, cytokeratin. HOMA-IR: homeostatic model of assessment insulin resistance. ROMs: reactive oxygen metabolites. TNF: tumor necrosis factor.
CHAI was positively correlated with:
- •
BMI (rs = 0.394; p = 0.004).
- •
Waist circumference (rs = 0.465; p = 0.001).
- •
HMW/total adiponectin ratio (rs = 0.306; p = 0.027).
- •
TNF-α (rs = 0.519; p = 0.001).
- •
ALT (rs = 0.687; p < 0.001).
- •
GGT (rs = 0.571; p < 0.001).
- •
Triglycerides (rs = 0.551; p < 0.001).
- •
Uric acid (rs = 0.290; p = 0.037).
- •
Glucose (rs = 0.594; p < 0.001).
- •
Insulin (rs = 0.856; p < 0.001).
CHAI was inversely correlated with:
- •
Total adiponectin (rs = −0.335; p = 0.015).
- •
HMW adiponectin (rs = −0.279; p = 0.045).
- •
HDL-C (rs = −0.410; p = 0.003).
- •
QUICKI (rs = −0.868; p < 0.001).
Notably, total and HMW adiponectin were highly correlated (rs = 0.947; p < 0.001) (Figure 2).
DiscussionIn this study, patients with either NAFL or NASH had lower total and HMW adiponectin and higher TNF-α levels compared to control group, and patients with NASH had higher CK-18 levels compared to either NAFL patients or controls. No difference in visfatin levels or HMW/total adipo-nectin ratio was found among the groups. Furthermore, CHAI was introduced, based on parameters being dif-ferent between NAFL and NASH patients.
It should be highlighted that CHAI is constituted by CK-18, HOMA-IR and AST, reflecting he-patocellular apoptosis, IR and hepatocellular dysfunction, respectively, meaning three major pieces of the pathogenetic puzzle of NAFLD.3 CHAI was escalating from controls to NAFL and NASH and was higher by increasing the severity of all main histological lesions (Table 3). Importantly, CHAI could distinguish NASH from NAFL non-invasively. However, despite its relatively high specificity, CHAI had low sensitivity; if it was translated in clinical terms, low CHAI levels may exclude NASH, but high CHAI levels cannot safely prove NASH. Furthermore, CHAI was correlated with anthropometric parameters (BMI, waist circumference), parameters of IR syndrome (triglycerides, HDL-C, uric acid, glucose), other hepatocellular parameters (ALT, GGT) and adipo-cytokines (total and HMW adiponectin and TNF-α). However, although these characteristics make CHAI an attractive non-invasive alternative, a validation study is needed before introducing CHAI in clinical practice. Non-invasive markers of NASH or specific histological lesions, including fibrosis, are a field of intensive research, intending to decrease the number of patients subjected to liver biopsy, which is currently the gold standard for the diagnosis of NAFLD. Previously introduced non-invasive markers are reviewed elsewhere13 and are beyond the scope of this study.
Serum CK-18 levels were higher in NASH patients compared with either NAFL patients or controls in this study, and CK-18 was higher by increasing the severity of lobular and portal inflammation. CK-18 is regarded as an index of hepatic apoptosis and is steadily higher in patients with NASH than NAFL,13 thereby indicating that hepatic apoptosis is intensified when NAFL progresses to NASH. CK–18 appears to be an accurate biomarker for NASH14 and it has been previously used in combined non-invasive markers for the differentiation between NAFL and NASH.15,16
Total adiponectin levels have been previously studied. In a meta-analysis of 27 observational studies with biopsy-proven NAFLD patients, total adiponectin levels were overal higher in controls than either NAFL or NASH, and lower in NASH than NAFL;17 Our study, was in line with this meta-analysis in terms of controls and NAFL or NASH comparisons, but not in terms of NAFL-NASH comparison; however, nine of 19 studies providing comparative data between NAFL and NASH groups in this meta-analysis showed no between group difference in total adiponectin levels. We have no solid explanation for this discrepancy, but population (gender, BMI and ethnicity distribu-tion)17 and methodological differences (sampling error, intra- and inter-observer variability, distinct commercial kits, distinct NAFLD classification sys-tems)17–19 could be speculated.
HMW adiponectin has been proposed to be the biologically more active form of adiponectin, and have a stronger association with IR and cardiovascular disease.20 However, in this study, HMW was not shown to be superior to total adiponectin: both total and HMW adiponectin levels were higher in controls than NAFLD, but similar between NAFL and NASH patients. Furthermore, total and HMW adiponectin levels were highly and linearly correlated each other (Figure 2). To our knowledge, there is only one previously published clinical cross-sectional study evaluating HMW levels in NAFLD. In line with our findings, higher total and HMW adiponectin levels were shown in controls, but similar between NAFL and NASH patients.21 Furthermore, no previous study has reported on HMW adiponectin in relation to liver fibrosis in NAFLD patients. However, given that HMW adiponectin measurement is technically more demanding and costly, it could not be currently recommended for NAFLD patients.
Serum visfatin levels were similar between NAFL and NASH patients in this study, as it was reported in two previously published studies with biopsy-proven NAFLD patients.22,23 Regarding controls, in the first study, no difference was shown, when the controls had BMI similar to NAFLD patients, but visfa-tin was lower in a second control group consisted of individuals with lower BMI.22 In the second study, visfatin was higher in the control group, but it consisted of individuals with lower BMI.23 Similar hepatic visfatin expression (immunohistochemically) was also shown between NAFL and NASH morbidly obese patients undergoing bariatric surgery; however, hepatic visfatin expression was higher in patients with liver fibrosis in this study.24 Collectively, although it seems that serum visfatin levels cannot be recommended for the non-invasive diagnosis of NASH, the local pathogenetic role of visfatin in the level of adipose and hepatic tissue needs further studies to be elucidated.
This study has certain limitations:
- •
The sample size was small; especially findings regarding the histological lesion of ballooning should be cautiously interpreted, because of skewed distribution.
- •
The controls were not subjected to liver biopsy, due to obvious ethical considerations.
- •
The cross-sectional design of the study cannot prove any causative relationship.
- •
Similarly, correlations cannot prove any causative relationship and they sometimes represent an epiphenomenon.
- •
Despite the statistically non-significant differences, there is a possibility of type II error given the overall small size of the three groups, which may be important for gender and BMI, given that they both could affect the adipocytokines levels.
- •
Regarding CHAI, a validation cohort is needed before introducing in clinical practice.
Conclusion
Patients with either NAFL or NASH had lower total and HMW adiponectin, and higher TNF-a levels compared to control group, and patients with NASH had higher CK-18 levels compared to either NAFL patients or controls. Importantly, CHAI index was introduced, constituted by serum CK-18, AST and HOMA-IR, reflecting hepatocellular apopto-sis, hepatocellular dysfunction and IR, respectively. CHAI was escalating from controls to NAFL and NASH and increased as the severity of all the main histolo-gical lesions of NAFLD advanced. However, a validation study is needed before introducing CHAI as a novel non-invasive index in clinical practice.
Abbreviations- •
ALP: alkaline phosphatase.
- •
ALT: alanine transaminase.
- •
AST: aspartate transaminase.
- •
BMI: body mass index.
- •
CHAI: CK-18, HOMA-IR, AST Index.
- •
CK: cytokeratin.
- •
GGT: gamma-glutamyl transferase.
- •
HDL-C: high-density lipoprotein cholesterol.
- •
HMW: high molecular weight.
- •
HOMA-IR: Homeostatic model of assessment -insulin resistance.
- •
IR: insulin resistance.
- •
NAFL: simple nonalcoholic fatty liver.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NAS: NAFLD activity score.
- •
NASH: nonalcoholic steatohepatitis.
- •
QUICKI: quantitative insulin sensitivity check index.
- •
ROMs: reactive oxygen metabolites.
- •
TNF: tumor necrosis factor.
None.