Objective. To evaluate alcohol use in patients with HIV infection, assess ethnic and social associations, and describe outcomes.
Material and methods. Design: cohort study. Setting: Academic HIV-Liver Clinic. Patients: 431 HIV-infected patients (371 men, 60 women); 249 patients with HIV/HCV coinfection, 115 HIV alone, and 67 with HIV/HBV. Intervention: alcohol use was estimated at first interview and reported as the estimated average lifetime consumption in grams/day. Outcome measures: laboratory values, liver fibrosis, decompensation and mortality.
Results. Twenty-two percent of patients in the entire cohort had high risk lifetime average alcohol consumption, defined as ≥ 50 mg/day. Fifty-six percent of patients had quit all alcohol when first evaluated, but follow-up showed that 26% continued high risk consumption. By univariate analysis high alcohol consumption was associated with Latino ethnicity, injection drug use (IDU) and hepatitis C (HCV) coinfection. Multivariable analysis showed only IDU to be independently associated with high alcohol consumption (RR = 4.1, p = 0.0005). There were no significant differences in laboratory values, including CD4 cell counts, except for a trend towards higher transaminases and liver fibrosis scores, between high and low alcohol users. All-cause mortality was statistically higher in the high (37%) vs. low (25%, p = 0.03) alcohol use group, and was associated with both IDU (RR = 2.2, p = 0.04) and the amount of alcohol consumed (RR = 1.1, p = 0.04). Liver decompensation and mortality were both higher in the high use group but of borderline significance. Using an ordinal grouping, we found a strong correlation (R = 0.88) between alcohol consumption and the percentage of liver death over total deaths, with lowest mortality rates found in those use of 10 g/day or less.
Conclusions. Unsafe use of alcohol is prevalent in HIV-infected patients and stoppage is not universal. There is a significant impact on all-cause mortality and a trend towards higher liver morbidity and mortality. IDU is significantly and independently associated with high ethanol intake. Practitioners should strongly recommend that HIV patients minimize alcohol use.
Alcohol use in HIV-infected patients has been associated with a number of medical and social issues, including noncompliance with antiretroviral therapy,1 risky sexual and needle-sharing behavior,1-4 and decreased cellular immunity to specific HIV antigens.5 Safe alcohol drinking is difficult to define, but there appears to be a threshold of alcohol consumption (estimated in drinks or grams per unit time) beyond which alcoholic liver disease or other disorders can develop.6-9 Unsafe alcohol use has been defined as ≥ 50 grams/day (g/d),8 > 30 g/d,7 but even lower consumption (23 g/d for women) have the potential for causing alcoholic liver disease (ALD).9 There is little data about alcohol and hepatic end-organ damage in HIV patients without viral hepatitis, and few data regarding its relationship to viral hepatitis B or C.10,11 In addition, the use of alcohol in minorities affected by HIV, particularly Latinos, has not been adequately addressed. In fact only two studies on alcohol in HIV disease included > 50% minorities.12,13
ObjectiveTo evaluate alcohol use in patients with HIV infection, with or without viral hepatitis, seen in an urban academic HIV-Liver Clinic.
Material and MethodsHIV-positive patients who were referred to the Los Angeles County HIV Hepatitis Clinic since January 1994 were evaluated. The clinic was staffed by the author and evaluated patients referred for elevated liver enzymes, hepatitis C, or hepatitis B. Serum liver enzymes, prothrombin time, HIV antibody with Western Blot confirmation, anti-HCV antibody and HBsAg were performed at the Los Angeles County/University of Southern California Medical Center Central laboratory as part of the routine evaluation of these patients. Anti-HCV or HCV RNA positivity defined the HCV group and HBsAg positivity defined the HBV group. Hepatitis Delta antibodies were obtained only in patients who tested positive for HBsAg.
Race-ethnicity was established patients at first interview by self reporting, African-American, Caucasian, Latino (or Hispanic) or other. Recorded risk factors for HIV infection were: men having sex with men (MSM), injection drug use (IDU), transfusion and unknown. The latter category included patients who denied all of the above risks.
Alcohol use was estimated at first interview (in English or Spanish as appropriate) as the average lifetime consumption in g/d. The amount was calculated by periods in the patient’s life starting with periods of daily drinking. Consumption of < 12 drinks per year was arbitrarily given a value of 0 g/d; 1 to 4 drinks/month was given a value of 5 g/d. Social consumption was arbitrarily defined as a maximum of 3 alcoholic drinks per week and was given a value of 10 g/d. Patients who could not quantify their heavy ethanol use were assigned the value for the 95% percentile for their diagnostic category (Table 1). We define high alcohol consumption as an estimated lifetime consumption of 50 g/day or greater for men and > 30 g/day for women.8
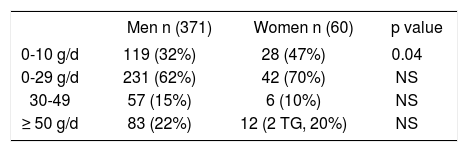
Patients’ drinking history divided into three strata according to estimated average lifetime ethanol consumption, comparing women and men.
| Men n (371) | Women n (60) | p value | |
|---|---|---|---|
| 0-10 g/d | 119 (32%) | 28 (47%) | 0.04 |
| 0-29 g/d | 231 (62%) | 42 (70%) | NS |
| 30-49 | 57 (15%) | 6 (10%) | NS |
| ≥ 50 g/d | 83 (22%) | 12 (2 TG, 20%) | NS |
TG: Transgender.
Of the 474 patients evaluated, 431 (91%) had data on alcohol use and therefore form the basis of this report. Liver biopsies were performed per standard of care, for the evaluation of patients with elevated liver tests with acceptable coagulation parameters. Fibrosis was scored from 0 (no fibrosis) to 4 (cirrhosis) according to published criteria.8 Severe liver disease was defined as either one of the following: death from liver disease, or cirrhosis with portal hypertension (splenomegaly) or decompensated liver disease (i.e. evidence of ascites or varices). Data on deaths was obtained from the Los Angeles County HIV Epidemiology Unit. Liver death was defined as any of the following being reported in the death certificate: cirrhosis, liver failure, alcoholic liver disease, hepatitis C, hepatitis B, hepatocellular carcinoma, hepatic encephalopathy.
The prospective collection of data for this study was approved by the LAC+USC Institutional Review Board.
StatisticsNon-parametric and χ2 tests were used as appropriate. P values were 2-tailed. A logistic regression model was developed with Statview Software 5.01 (SAS Inc., 3rd. Ed. 1999) and was used to assess the association between different variables and high alcohol usage.
ResultsThe study followed 431 patients for a median of 6 years; 371 (84%) male and 60 (16%) female (of which two were transgender) patients. The median age was 39 (range 21-65) and 36 (20-58) years in male and female patients, respectively (p = 0.08).
African Americans (18% women) comprised 23% of patients, Caucasians (4% women) 27%, Hispanics (17% women) formed 47% of the cohort. Asians and other race-ethnicities made up 1% and 2% of the cohort, respectively.
249 patients had HIV + HCV coinfection (hepatitis C, including coinfection with HBV and/or HDV), 115 had HIV alone, and 67 had HIV + HBV (hepatitis B, with or without Delta hepatitis but with a negative HCV RNA).
Women were more likely than men to report a lifetime ethanol consumption between 0-10 g/d (p = 0.04) (Table 1). Median lifetime ethanol use was 10 g/d in women, and 18.5 g/d in men (p = 0.004). However, because in women a lower cutoff (30 g/d) has been suggested as a ‘safe’ consumption,9 using that cutoff, women were actually more often in the ‘unsafe alcohol use’ category (30% ≥ 30 g/d), although not statistically different from men (22% ≥ 50 g/d). Because of this, and to prevent a decrease in number of patients, only the 50 mg/day cutoff was used to define high alcohol consumption in subsequent analyses.
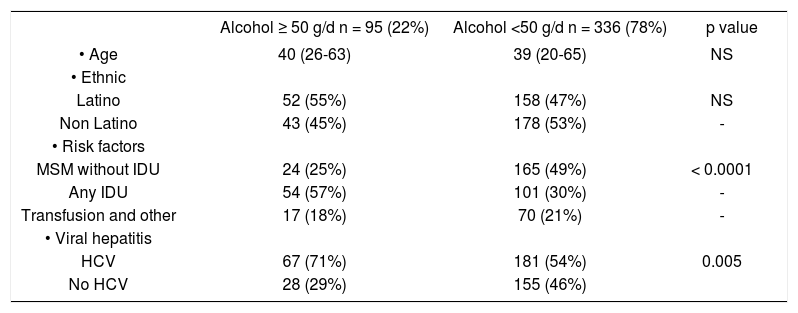
Univariate analysis showed that a lifetime alcohol use ≥ 50 g/d was more likely to be associated risk factors for HIV (MSM vs. IDU vs. transfusion), and viral hepatitis C (Table 2). Patients with a high alcohol consumption (n = 95, 22%) were older and were more often self-categorized as Latinos, but these differences were not statistically significant. Using a logistic regression model with age, gender, ethnicity, risk factors for HIV acquisition, viral hepatitis coinfection, and CD4 counts as independent variables, only injection drug use was strongly and independently associated with a lifetime average ethanol use equal or > 50 g/d (RR = 4.1, 95% CI 1.9-7.3, p = 0.0005). Alcohol use ≥ 50 g/d wasn’t associated with significantly different CD4, CD8 (not shown), and number of HIV medications or opportunistic infections. However heavy alcohol use was marginally associated with higher
Demographic and laboratory values in HIV-positive patients with high (≥ 50 g/d) vs. low lifetime ethanol use.
| Alcohol ≥ 50 g/d n = 95 (22%) | Alcohol <50 g/d n = 336 (78%) | p value | |
|---|---|---|---|
| • Age | 40 (26-63) | 39 (20-65) | NS |
| • Ethnic | |||
| Latino | 52 (55%) | 158 (47%) | NS |
| Non Latino | 43 (45%) | 178 (53%) | - |
| • Risk factors | |||
| MSM without IDU | 24 (25%) | 165 (49%) | < 0.0001 |
| Any IDU | 54 (57%) | 101 (30%) | - |
| Transfusion and other | 17 (18%) | 70 (21%) | - |
| • Viral hepatitis | |||
| HCV | 67 (71%) | 181 (54%) | 0.005 |
| No HCV | 28 (29%) | 155 (46%) |
MSM: Men having sex with men. IDU: Injection drug use. HCV: Hepatitis C virus. HBV: Hepatitis B virus.
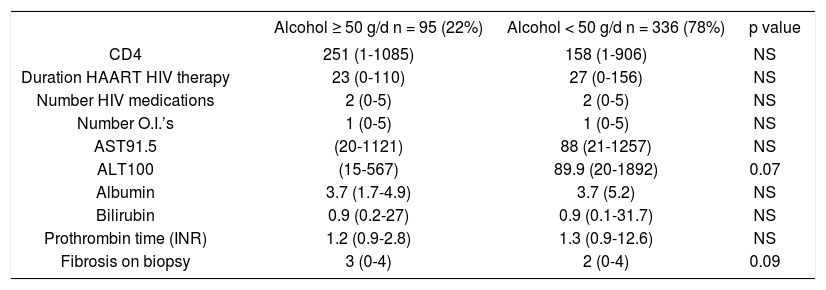
ALT (p = 0.07) and higher median hepatic fibrosis (p = 0.09) (Table 3).
Median (range) laboratory data in HIV-positive patients with high vs. low lifetime ethanol use.
| Alcohol ≥ 50 g/d n = 95 (22%) | Alcohol < 50 g/d n = 336 (78%) | p value | |
|---|---|---|---|
| CD4 | 251 (1-1085) | 158 (1-906) | NS |
| Duration HAART HIV therapy | 23 (0-110) | 27 (0-156) | NS |
| Number HIV medications | 2 (0-5) | 2 (0-5) | NS |
| Number O.I.’s | 1 (0-5) | 1 (0-5) | NS |
| AST91.5 | (20-1121) | 88 (21-1257) | NS |
| ALT100 | (15-567) | 89.9 (20-1892) | 0.07 |
| Albumin | 3.7 (1.7-4.9) | 3.7 (5.2) | NS |
| Bilirubin | 0.9 (0.2-27) | 0.9 (0.1-31.7) | NS |
| Prothrombin time (INR) | 1.2 (0.9-2.8) | 1.3 (0.9-12.6) | NS |
| Fibrosis on biopsy | 3 (0-4) | 2 (0-4) | 0.09 |
O.I.’s: Opportunistic infections.
The clinical impact of lifetime alcohol consumption using all-cause mortality, liver mortality and clinical decompensation as endpoints is shown in table 4. Although higher all-cause mortality rates were noted in the high alcohol category, liver-specific mortality did not achieve statistical significance (p = 0.18). Multivariable analysis of all-cause mortality, including viral hepatitis, alcohol use, risk behavior, ethnicity and gender indicated a modest correlation with IDU (RR = 2.2, p = 0.04) and alcohol use (RR = 1.1, p = 0.05). Multivariable analysis of liver deaths revealed no significant independent association, likely due to small numbers. However, using alcohol consumption as an ordinal variable, we found a strong correlation (R = 0.88) between alcohol use and liver mortality. The percentage of liver mortality over total deaths was lowest in those with > 10 g/d (13%) vs. 26% in those reporting 11-30 g/d, 25% in the 31-50 group and 40% in those with higher intakes.
Of the 95 patients who had a lifetime history ≥ 50 g/d, 43 (45%) had data on changes in alcohol usage. Twenty-four of those 43 (56%) had stopped completely for a median duration of one year (range 1 month-14 years). Eight (18%) reported significant decreases (< 30 g/day), and 11 (26%) had ongoing high risk usage.
DiscussionThere is no single agreed-upon instrument for measuring alcohol consumption in humans. Most estimates are believed to underreport actual consumption.14 Despite these difficulties, a quantitative assessment of ethanol consumption is important from at least two points of view. First, using a behavioral approach, it has been demonstrated that a consumption > 23 and 31 g/d (in women and men respectively) was associated with problem alcohol use.15 Secondly, alcohol has a dose-related association with end-organ damage. In a large populationbased Danish study, the estimated alcohol usage did correlate with the likelihood of a diagnosis of ALD at discharge from the hospital.9 In a cohort of hospitalized patients in Italy, ethanol consumption was associated with decompensated cirrhosis, and this effect was directly related to the estimated lifetime quantity of alcohol consumed.16 This underscores the importance of estimating quantity-frequency ethanol usage, as the latter is related to both liver disease9,16 and ‘social problems’.15
This report estimates the average lifetime ethanol use in a cohort of HIV-infected patients followed in an urban academic publicly funded HIV Clinic, serving primarily underinsured and uninsured patients. Although we used no standardized questionnaire, the data we obtained are in keeping with published data. The percentage of men and women drinking 0-10 g/d was 30 and 77% respectively, in a population-based Danish study,9 compared to 32 and 47% in our study. Likewise, use ≥ 50 g/d was 20 and 2% in men and women9 compared to our cohort, 22 and 20% respectively. Our alcohol consumption estimates are in the general range of previously published reports.1,12,13,17-19,19a Thus, we believe that our ethanol estimates are reasonable for the purpose of this analysis. We used 50 g/d as the threshold for a high alcohol use, because it has been shown to be associated with faster fibrosis progression in chronic hepatitis C,8 although it is possible that lower thresholds may also be clinically relevant.7,9,19b Overall, 22% of our cohort had a high estimated lifetime alcohol use, as defined. Women in our cohort had substantial rates of unsafe use (30% had > 30 g/day) compared to only 12% in the Johns Hopkins HIV Clinical Cohort (> 28 g/day).19b Interestingly in the latter cohort, men had actually lower rates (< 10%) of hazardous drinking compared to females. Auspiciously, in our group of patients, > 50% of those interviewed reported a stoppage of alcohol use. Unfortunately, 26% patients acknowledged continued usage at levels > 50 g/d. The remainder reported a continued use, but decreased to lower levels. In view of the facts that the peak age for ethanol dependency is known to occur in the mid-30’s,6 and that our group’s median age was 39 years, finding a decrease in recent alcohol usage was not surprising. Others, using DSM definitions, not quantity-frequency instruments, also showed that ethanol dependency in HIV patients decreased over time.17-19 A recent article looked at alcohol consumption in the past 30 days in HIV patients found no impact on fibrosis (estimated by APRI) but made no attempt to quantify past alcohol exposure, which may be much more relevant for liver fibrosis than current use.20
Women were more likely than men to drink 10 g/d or less; however, similar percentages of men and women drank ≥ 50 g/d. In fact, using a lower cutoff for unsafe drinking,9,15 women had a higher percentage of individuals potentially at risk for ‘problems’ or ALD (≥ 30 g/d, 30%), although not statistically different from men (23% ≥ 50 g/d).9,15 Although Latinos and patients with HCV coinfection were more likely to be in the high usage category, multivariable analysis did not identify either gender, ethnicity or viral hepatitis coinfection as independent variables associated with alcohol use. This finding may be at odds with published data in the general (non-HIV) population, where frequent heavy drinking is more common in African Americans and in Lati-nos.21
There was a strong and independent association between IDU and high ethanol use. This is not surprising in view of the substantial percentage of patients enrolled in methadone programs who are reported to have alcohol problems, pointing to poly-substance abuse.1,22 Among MSM, heavy drinking (≥ 50 g/d) was identified in 15% of those without IDU, and in 28% of those who also reported IDU. Three studies, all in HIV-positive IDU patients, strengthen our finding of an association between IDU and alcohol in HIV-positive patients because 33% to 68% of patients drank > 40 g/d.10,12,13 In non-HIV patients, the association between alcohol and illicit drug use is also well described.1,22 To our knowledge, this is the first cross sectional study in a large cohort of HIV patients, unselected on the basis of risk factors and hepatitis coinfection, that unequivocally associates ethanol and IDU. The latter association also explains the higher percentage of patients with HCV in the high ethanol group.
In this cohort, laboratory parameters were not different in those with less than or greater than 50 grams ethanol/day. In particular, the peripheral CD4 lymphocyte counts were similar in patients with low and high ethanol use. This may mean that in vivo alcohol has minimal effects on CD4, as was suggested by the MACS cohort evaluation.10,23 However, it is also possible that only recent alcohol use is associated with CD4 counts declines,5 as sobriety leads to increased CD4, in both HIV-positive and HIV-negative alcoholics.24 Since most of our patients had decreased or stopped alcohol use, our data do not rule out an important effect of alcohol on CD4 counts.
In terms of clinical consequences, there was a trend towards higher hepatic fibrosis scores in HIV positive patients with high alcohol consumption history. All-cause mortality was higher in those with high alcohol usage (p = 0.03). Liver mortality was significant (27% of all deaths) in patients with alcohol consumption < 50 g/d. However this percentage was higher yet in those with higher consumption (40%), but did not achieve statistical significance, likely due to the small numbers. Multivariable analysis showed that IDU and estimated lifetime alcohol use were the two variables associated with all-cause mortality, albeit of borderline statistical significance (p = 0.04). Progression of HIV to AIDS due to alcohol abuse has never been shown in a cohort study, and our data did not allow us to observe such progression.1,22
Interestingly, when we divided patients according to several strata of alcohol consumption, we found that there was a strong correlation (R = 0.88) between alcohol use and liver death as a percentage of all deaths. The liver death percentage jumped briskly above a consumption > 10 g/day. We suggest than in HIV patients the cutoff for harmful alcohol consumption is lower than previously published.8
This study suffers from several weaknesses. First, relatively few liver biopsies were performed. Thus, we cannot exclude the possibility that high alcohol use affects fibrosis. However, we were not able to demonstrate a major effect on liver mortality either. We have no data on follow-up of IDU; it would have been interesting to document whether or not IDU patterns also change over time, together with alcohol use. We also were unable to perform socioeconomic and psychiatric evaluations, including depression scores, and correlate those with alcohol consumption.
ConclusionIn summary, a lifetime average ethanol consumption ≥ 50 g/d is found in 22% HIV patients in a large urban HIV Clinic. However, we also show that > 50% of those patients have stopped all ethanol when first seen by a hepatologist. IDU is independently associated with high ethanol intake, while ethnicity and viral hepatitis C are most likely secondarily related to IDU. Although we found similar albumin, AST, CD4 and liver fibrosis scores in different alcohol consumption groups, this data should not be construed as evidence that alcohol consumption is acceptable, as we did not measure its impact on relationships, drug adherence, risky behavior or social issues. In fact, there was a definite association between high alcohol use and all-cause mortality, as well as trend for greater liver morbidity and mortality. This relatively high prevalence of potentially unsafe alcohol consumption in subgroups of HIV patients warrants:
- •
Further study of immunological and hepatic outcomes.
- •
Alcohol counseling, particularly in women and patients with polysubstance abuse.