We assessed the anti-fibrotic effects of methanolic black bean extract antioxidants in a carbon tetrachloride (CCl4) liver injury model in rats. Experimentally intoxicated animals received CCl4 for eight weeks, the reference and test groups received daily intragastric quercetin or daily intragastric black bean extract. Liver fibrosis was assessed and quantified using morpho-metric analysis. Expression of fibrosis related genes was measured by real time RT-PCR. Qualitative and quantitative histological analysis showed that administration of 70 mg/kg b.w. of black bean extract reduced hepatic fibrosis index by 18% compared to positive controls (P 0.006), as a result of a decrease in type I (44.3% less, P 0.03) and type IV (68.9% less, P 0.049) collagen gene expression compared to CCl4-injured and Quercetin treated rats. In conclusion, we provide evidence that this methanol black bean extract ameliorates liver fibrosis and types I and IV collagen gene expression, in the animal model used.
Practical applications: The compounds contained in this black bean extract exhibited strong antifibrotic effects in the CCl4 chronic liver injury model used; considering that this compounds are contained in a leguminous that has been used in human diet for a long time, their toxic potential should be very low, and this characteristic should favor their potential use in some other chronic or degenerative states that include an increase in inflammation and oxidative burst in their pathogenesis. Another possible application of this kind of extract could be its use as an antimicrobial or even antiparasitic therapeutic agent, although it is purely speculative.
Hepatic cirrhosis or liver fibrosis is known to be an irreversible distortion of the normal tissue architecture; this alteration develops during chronic liver damage.1 Multiple processes are involved in the progress of this lesion including: Oxidative stress by free radicals (FR); chronic inflammation mediated in part by the release of pro-inflammatory cytokines from Kupffer cells; and fibrosis induced by the paracrine action of pro-inflammatory and pro-fibrogenic cytokines produced by Kupffer cells and hepatocytes on hepatic stellate cells.2-5 As fibrosis progresses, quantitative and qualitative changes in extracellular matrix (ECM) composition take place. Total collagen and non-collagen ECM components increase 3 to 5 fold, and the ECM in the sub-endothelial space changes from a normal low density basement membranelike matrix, to an interstitial-type matrix rich in collagen fibrils.6 Type I collagen is the most abundant ECM protein in hepatic fibrosis, followed by type IV collagen. Therefore, the regulation of type I and IV collagen genes expression is crucial to understand the pathogenesis of this disease.7
Over the years, many compounds have been studied as possible protectors for liver cirrhosis, like antioxidants, such as flavonoids and other phenolic derivates. These compounds belong to a group of a bigger family known as phytochemicals. They are widely found in fruits and vegetables and while not having an energetic value, are an important part of the human diet.8 Flavonoids exhibit a wide variety of biological properties, including hepato-protection and inhibition of fibrosis.9
Beans are grains that have a high nutritional value due to their protein content. They also contain an elevated concentration of complex carbohydrates, soluble fibers, essential vitamins, metals and polyphenols,10,11 such as flavonoids and isoflavones.12 Moreover, dry beans exhibit strong antioxidant properties. Hence, it would be important to expand methods to assess the components which present antioxidant and anti-fibrotic activity.13 In recent studies, the effects of quercetin as an antioxidant have been demonstrated in several models of CCl4 liver injury.14
The objective of this investigation is to evaluate the anti-fibrotic effect of a black bean extract, rich in flavonoids and poliphenolic compounds, in a rat model of liver cirrhosis induced by CCl4.
Material and methodsPerla black blean:The Perla black bean was grown and cropped in the ITESM Agricultural Experimental Field in Linares, Nuevo León, México, during the 2004 spring-summer cycle, in rainfall plus supplementary irrigation. The soil was fertilized at the moment of planting with urea and triple superphosphate. No pesticides were used during the cycle.
Antioxidant extractionA total of 0.1 g of crude biomass obtained from seed coats of Perla black bean was dissolved in 400 mL of H2O, and 100 g of NaCl was added to this solution. The mixture was filtered and collected in an Erlenmeyer flask, where 200 mL of butanol were supplemented and left at room temperature for 30 min in order to achieve a full separation of the organic and aqueous phases. The organic phase was distilled and the butanol recovered. This organic phase was re-suspended in a 2:1 (v/v) solution of ethyl acetate and methanol, and the salts fully precipitated with 100 ml of ethylic ether. Furthermore, the solution was evaporated until mass dryness was obtained.
Antioxidant preparationFrom the latter extraction process, the remaining extract was dissolved in physiological saline solution, adjusting to final dose of 70 mg/kg b.w., according to the protocol.
Animals and groupsMale Wistar rats (Harlam, Mexico) were housed according to the Animal Care Protocol established by ITESM. Study animals (80 grams) received three intraperitoneal (i.p.) doses of 1 ml (Monday, Wednesday and Friday) of a 1:6 (v/v) mixture of CCl4 and mineral oil on the first week; three doses of a 1:5 mixture on the second week, three of a 1:4 mixture on the third, and three doses of a 1:3 mixture each week on weeks 4 to 8 (15). Four groups of 6 animals were studied. The reference group received CCl4 i.p. as explained above, and was concomitantly treated with daily intragastric quercetin at 70 mg/ kg b.w. daily for eight weeks; the test group received CCl4 and daily intragastric black bean extract at 70 mg/ kg b.w. daily for eight weeks. The positive control group received CCl4 and intragastric saline solution during the same period. The normal group did not receive CCl4, quercetin, nor black bean extract. Surviving animals in all groups were euthanized at the end of the eight week treatment period, two days after the last dose, and their livers were dissected and sectioned. One piece was immediately frozen and stored for RNA extraction, and another section was fixed in 10% formalin.
Morphologic analysisSections (approx 0.5 cm3) from the right median and left lobes of each rat liver were fixed and stored in 10% formalin at room temperature. Sections were treated using a Tissue-Tek II automatic processor and then embedded in paraffin. Paraffin blocks were cut using a Tissue-Tek Accu-Cut Microtome and 4 mm sections were stained using the Masson Trichromic technique. Images were obtained using an Olympus video-microscope, and a quantitative analysis was done using the Image-Pro Plus software, version 3.0 (for Windows); 20 random fields were analyzed per slide, and the ratio of connective tissue to the whole area of the liver was calculated.16 The sections were analyzed by 2 independent pathologists.
RNA IsolationTotal RNA was extracted from liver samples using TRIzol (Invitrogene Life Technology, Aukland 1135, New Zealand). The tissue was homogenized to 8000 rpm (Tissue Tearor 985370-14, Biospec Products, Inc.) in 500 ¡uL TRIzol (TRIzol reagent®, Molecular Research Center, Inc) and RNA was isolated by chloroform/isopropanol/ ethanol extraction,17 total RNA was treated with DNase-I, amplification grade (Invitrogen). RNA concentration and purity was tested by spectrophotometry at 260 and 280 nm (GeneQuant-pro, Amersham Bioscience).
Two-step real time RT-PCRTotal RNA isolated from each rat liver sample was reverse transcripted using Moloney Murine Leukemia Virus Reverse Transcriptase and random primers, according to the manufacturers protocol (Invitrogen MMLV Reverse Transcriptase, Part No. 28025.PPS). Forward and reverse LUX primers were designed using Primer Express Software (Invitrogen Life Technology) for S-Actin (housekeeping) forward sense TC-CTAGTCTCAATACGCAG and reverse CGCTCTAT-CACTGGGCATTGG, for type I collagen gene forward sense GTTTCAGTGGTTTGGATGGG and reverse CT-GCCAGGCTCTCCCTTAGGAC, and for type IV collagen gene forward sense CCTGCACTTGTAAACAT-AAG and reverse TGTTCACAGTCAAACCACTGCT.18 Target cDNA sequences were amplified by quantitative PCR using a fluorescence-based real-time detection method (Rotor gene RG 3000, Corbett Research, Australia). A volume of 12.5 uL of Platinum Quantitative PCR Super Mix-UDG (Invitrogen Life Technology) was used, 2 uL of cDNA, and molecular biology grade water to a total volume of 20 uL per reaction. PCR conditions were: initial temperature of 50°C for 2 minutes and 95°C for 2 minutes followed by 45 cycles of 95°C for 5 seconds and 60°C for 10 seconds. These conditions were obtained from the Invitrogen protocol for the Corbett Rotor Gene 3000 LUX primers.
Relative amounts of target genes were calculated using the comparative 2AACT method with S-actin as an endogenous reference and RNA pooled from livers of normal rats as a calibrator.
Statistical analysisNumerical data are expressed as mean ± S.D. differences between experimental groups were tested using ANOVA, and when appropriate, one-tailed Student’s t-test. Differences of proportions were assessed by chi-square analysis. Statistical significance was considered for p values < 0.05.
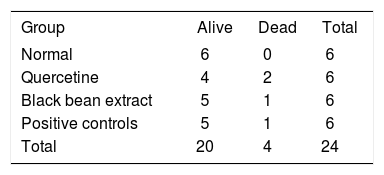
ResultsWe did not observe any statistically significant differences in the mortality nor survival rates among groups (Table I).
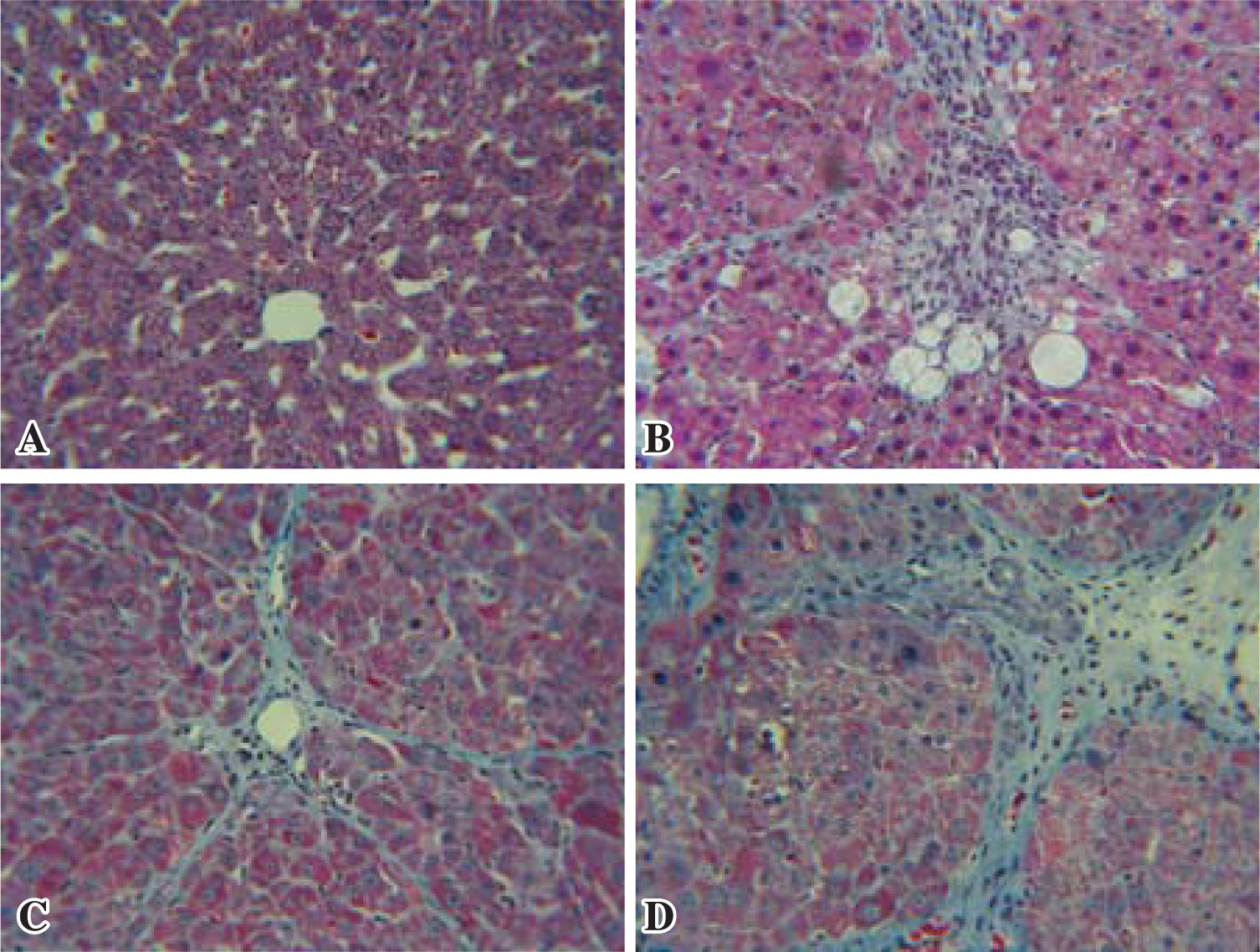
Histopathological changesAnimals in the normal group showed normal hepatic lobular architecture with central veins and radiating hepatics cords. The positive control, the reference and the test groups all showed evidence of pathological changes, including interstitial fibrosis, inflammatory infiltration, collagen deposition and steatosis. Histological analysis showed that the test group presented a significant reduction of collagen deposition (Figure 1).
Histopathological changes. Representative microscopic photographs, liver stained with Masson Tri-chromic, 40X. A: Normal rat liver, B: Reference (Quercetin), C: Test (Black bean extract), D: Positive control (CCl4). Black bean extract prevented the increase in extracellular matrix C, while the increased distortion of hepatic architecture in the positive group D is evident.
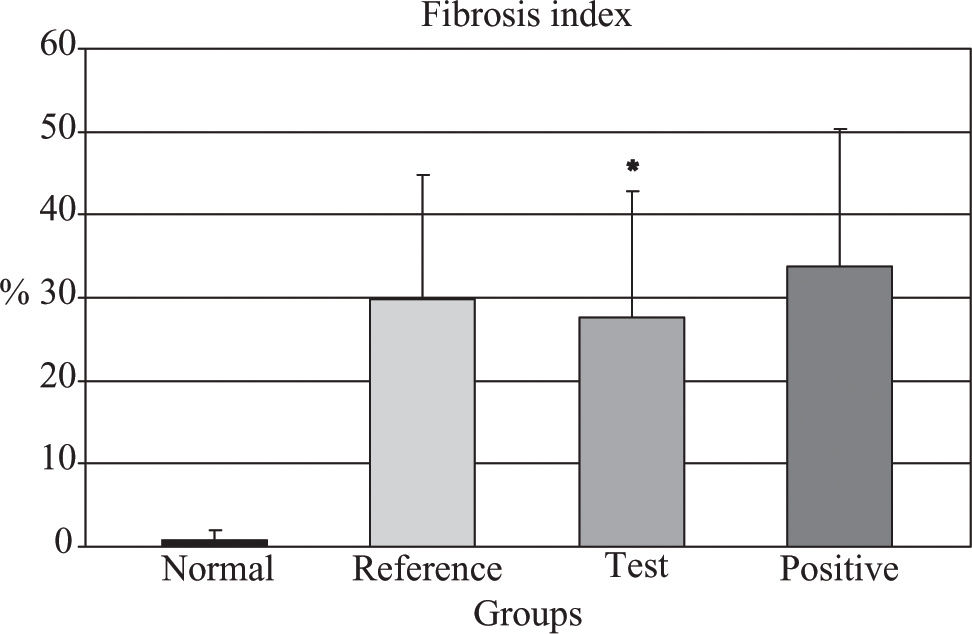
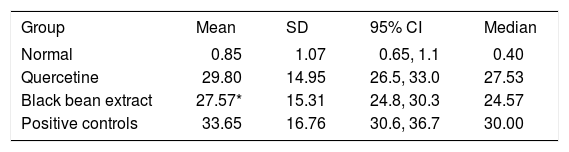
Quantitative analysis showed that the test group achieved an 18% fibrosis reduction when compared to positive controls (p = 0.006) (Figure 2). The reference group had no significant reduction in fibrosis. The data are showed in Table II.
Fibrosis index (%).
| Group | Mean | SD | 95% CI | Median |
|---|---|---|---|---|
| Normal | 0.85 | 1.07 | 0.65, 1.1 | 0.40 |
| Quercetine | 29.80 | 14.95 | 26.5, 33.0 | 27.53 |
| Black bean extract | 27.57* | 15.31 | 24.8, 30.3 | 24.57 |
| Positive controls | 33.65 | 16.76 | 30.6, 36.7 | 30.00 |
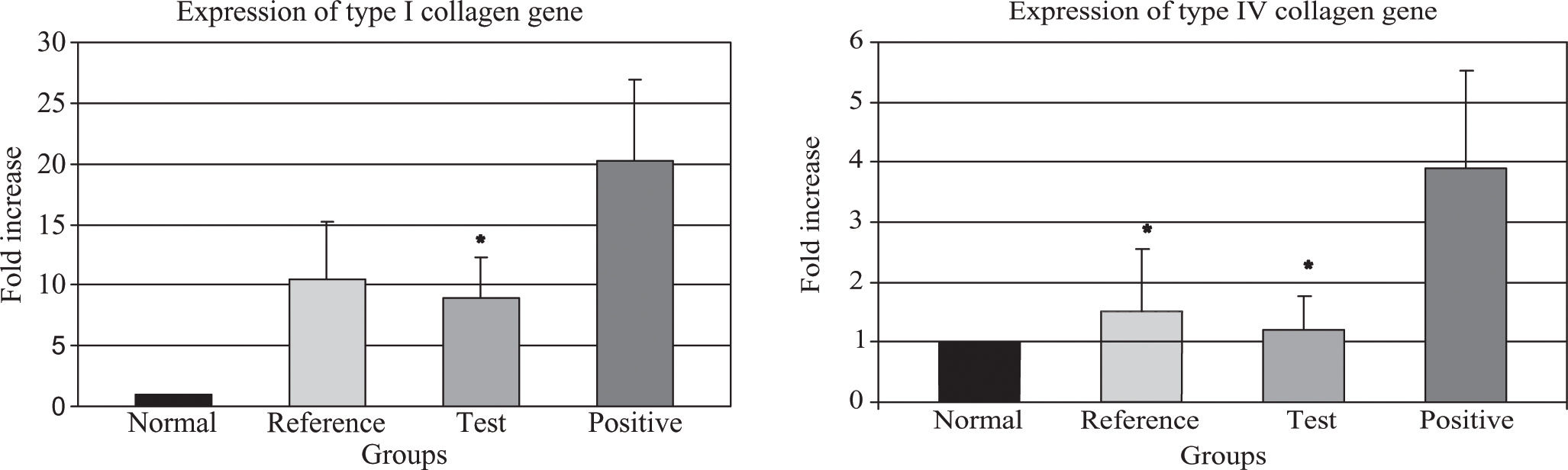
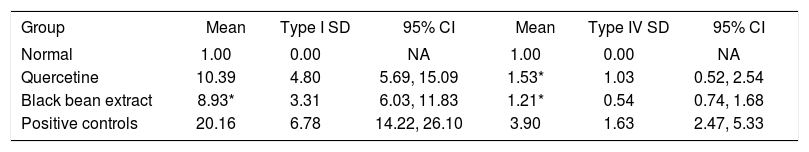
The data regarding type I and IV collagen gene expression are depicted in Table III. Test group showed a decrease of 44.3% in type I collagen gene expression, compared to the positive group (p = 0.030), (Figure 3-A).
Fold increase in collagen genes expression.
(A, B). Q-PCR analysis of the expression of types I and IV collagen genes. (A) Transcript levels of type I collagen gene (*) p < 0.05, test group compared against positive group; and (B) type IV collagene gene (*) p < 0.05, test and reference group compared against positive group; from the same Q-PCR run using the 2-AACT method. T-test for independent groups, error bars indicate standard deviation.
When we evaluated the expression of type IV collagen gene, the test group had 68.9% less than the positive control group, (p = 0.026) which is statistically significant, while the reference group also showed a significant decrease (60.8% less) compared to the positive control group (p = 0.049) a difference that is barely significant (Figure 3-B).
DiscussionCarbon tetrachloride under laboratory conditions is one of the most common and widely used liver intoxicators today.19 Its action is based on membrane lipid peroxidation and induction of trichloromethyl radical (.CCl3), resulting in severe cell damage.20 Antioxidants represent a new perspective in liver injury and fibrosis prevention.21 Previous studies have shown that antioxidants, including naringenin, N-acetylcysteine, vitamin E, sylimarin, quercetin and rhein decrease lipid peroxidation and partially ameliorate liver injury.10,22-26 Extracts obtained using 100% methanol as solvent have demonstrated to possess two of the most important families of antioxidants: anthocyanins and tannins.26,27
Hepatic fibrosis is usually initiated by hepatocyte damage, leading to recruitment of inflammatory cells and platelets, activation of Kupffer cells and subsequent release of cytokines and growth factors.28 These factors probably link the inflammatory and repair phases of liver cirrhosis by activating hepatic stellate cells (HSC). Previously HSC have been identified as an important cellular source of extracellular matrix (ECM) in liver fibrosis.7 The activated HSC undergo a phenotypic transdifferentiation to contractile myofibroblast expressing a-smooth muscle actin and ECM.19 It has been shown that lipid peroxidation products induce genetic overexpression of fibrogenic cytokines and increase the synthesis of collagen and initiate the activation of HSC.28
In our experiment, the use of a black bean extract belonging to a cultivar previously characterized as containing a high concentration of antioxidants29 significantly decreased the expression of type I and IV collagen genes and probably also diminishes the activation of HSC; partially protecting the liver from the CCl4 induced fibrotic effect. Rats treated with the extract had reduced liver fibrosis by histological examination and a lower expression of type I and IV collagen genes. This effect could be mediated by means of lack of activation of profibrogenic citokine TGFβ, which in turn would not be acting as an activator of the SMAD signaling pathway in HSC, and thereafter the stimulatory effect of this pathway on types I and IV collagen genes would be diminished.30,31 Our results follow the same pattern as the results published in previous works with the use of quercetin, salacia reticulata and rhein.14-25-26 Since this extract contains a myriad of compounds, and among them is quercetin, we hypothesize that the increased antifibrotic effect found, may be due to the sum of the action of all the flavonoids contained.
Moreover, the cellular response to polyphenols involves mainly cell surface receptors and key enzymes as transducers for intracellular signaling, and not a direct physical contact with free radicals. Under such circumstances, the change in redox potential supposedly induced by our extract would imply a modification of the redox state of the hepatocytes, thereby generating changes in the reactions dependent upon oxidation-reduction,32 although we did not directly measure this redox potential, and therefore can not assume this as true.
ConclusionThe use of a black bean extract can decrease the expression of type I and IV collagen genes, and reduce the hepatic fibrotic index in a model of CCl4 induced liver fibrosis. Our data warrant further investigation of this extract as a potential treatment in other models of hepatic fibrosis and in the clinical setting.
Competing interestsThere were no financial or any other competing interest involved in this study.
Authors contributionsALR conceived and participated in the design of the study, helped and supervised all test and analysis during study, and participated in the writing and corrections of this paper. NAC carried out RNA real time PCR and performed CCl4 administration to the study rats. BC carried out the antioxidant extracts administration to the study rats. VJLD performed statistical analysis and participated in the writing and corrections of this paper. GEGS performed the organ extraction. JFI performed antioxidant extraction system. VMO participated in the RNA extraction and the real time PCR. LMG participated in the organ extraction and participated in the writing of this paper. GJ and JFGG performed histological preparations and histological analysis. JEMC supervised and participated in the design of the study and the writing of this paper.
AcknowledgementsWe express our deepest gratitude to Sergio Serna-Saldivar, PhD, who kindly provided us with the standardized extract for this research.
We are indebted to the animal care facilities of the ITESM School of Medicine in charge of Dr. Luis Vazquez-Juarez, for all his support to this project.