Rituximab is a chimeric anti-CD20 monoclonal antibody that is a widely used for the treatment of B cells non-Hodgkin lymphoma. The use of chemotherapy regimens containing rituximab in HCV-positive patients with non-Hodgkin lymphoma has been associated with liver dysfunction, but no cases of cholestatic hepatitis C were described. To our knowledge, this is the first case of cholestatic hepatitis C in an HCV-positive patient with diffuse large B-cell lymphoma describes in the literature. We discuss the pathogenetic mechanisms underlying this severe form of hepatitis and describe its evolution after antiviral treatment.
Cholestatic hepatitis C was first described in liver transplant (LT) patients, and it is characterized by inflammatory infiltrates on liver biopsy, hepatocyte ballooning, severe cholestasis and a rapid evolution to liver fibrosis.1 This condition is rapidly progressive and may result in allograft failure within months of its onset. Moreover, it is usually resistant to antiviral treatment. Several cases of cholestatic hepatitis C, occurring as a consequence of posttransplantation immunosuppression regimens, have been described in the literature.2,3 Liver damage following chemotherapy containing rituximab regimens has been associated with reactivation of hepatitis C virus (HCV) infection in a variable percentage (3.7-20%) of HCV-positive patients with non-Hodgkin lymphoma (NHL), but not cases of cholestatic hepatitis C were described in the literature.4–6 Here, we describe the first case of cholestatic hepatitis C that occurred in an HCV-positive patient with diffuse large B-cell lymphoma (DLBCL) after chemotherapy containing rituximab. We also discuss the pathogenesis of this condition and describe its evolution after antiviral treatment.
Case ReportA 67-year-old Caucasian man who had been positive for HCV since 2005 with consistently normal alanine aminotransferase (ALT) levels controlled over time, was referred to our hospital in April 2010 because of low-grade fever and cough. He had a history of arterial hypertension, which was treated with antihypertensive drugs; furthermore he presented in the past recurrent episodes of chronic sinusitis and bronchitis and two episodes of lung pneumonia due to Haemophilus influenzae. HCV RNA level was 120,000 IU/mL, and the HCV genotype was 2a/2c. He was also positive for anti-hepatitis B surface antigen (anti-HBsAg) and negative for anti-hepatitis B core antigen owing to previous vaccination. His alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were normal at baseline (19 and 18 IU/mL, respectively). Positron-emission tomography/computed tomography (PET/CT) revealed a lesion in the left pulmonary lobe associated with mediastinal and abdominal lymphadenopathy. A biopsy of the pulmonary lesion was performed. Histological examination of the biopsy specimen revealed a DLBCL, with 80% Ki 67 index of proliferation as marker of elevated cell proliferation. Bone marrow biopsy was normal. The patient was found to have stage IV NHL. The absolute lymphocyte counts at baseline were normal: CD3 lymphocytes, 1,900 cells/uL (normal, 6902,540 cells/uL); CD4+ cells, 920 cells/uL (normal, 410-1,590 cells/uL); CD8+ cells, 780 cells/uL (normal, 190-1,140 cells/u L); and B lymphocytes, 250 (normal, 90-660 cells/u L). A low value of total Immunoglobulin was noted (600 mg/dL) but this value was referred from the patient to be lower in previous controls. Before starting chemotherapy, a transient elastography (FibroScan Echosens Paris France) was performed and yielded a value of 4.1 kPa, indicating mild liver fibrosis.
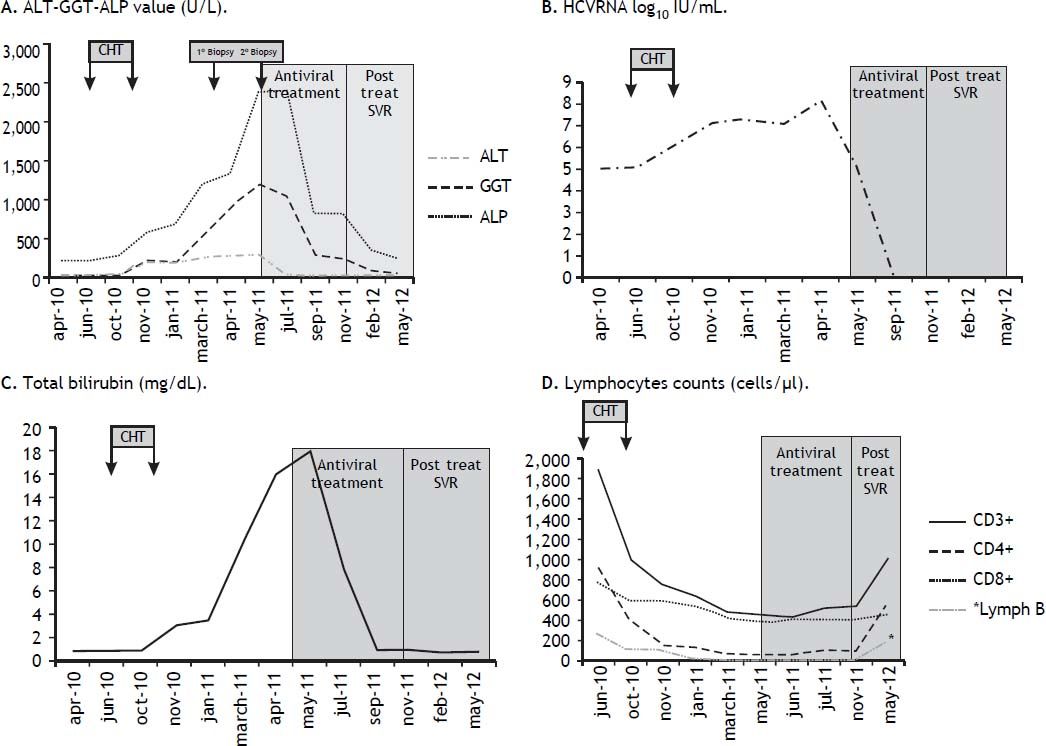
In June 2010, the patient started chemotherapy with cyclophosphamide, vincristine, prednisone plus rituximab (CVP-R), and this treatment ended in October 2010 after a total of six cycles of therapy. Restaging with PET/CT at the end of chemotherapy showed a complete hematological response. During chemotherapy, the ALT and AST levels were normal, while a slight increase in gammaglutamyl transpeptidase (GGT) was noted. The serum HCV RNA level increased to 1.5 million IU/mL (Abbott Illinois USA). In November 2010, liver biochemistry showed an increase in ALT (four times the normal value; x4 N) and AST (x4 N) associated with cholestasis (GGT, x4 N; total bilirubin, x3 N; alkaline phosphatase [ALP], x2 N) and HCV RNA level increased to 15 million IU/mL. Liver ultrasonography showed no dilatation of the biliary tree, along with acalculous cholecystis, homogeneous hepatomegaly and normal margins. A liver biopsy was recommended, but the patient refused it. We began therapy with ursodeoxycolic acid at a dose of 1,350 mg/day per os. In March 2011, a considerable increase in cholestasis was noted (total bilirubin, x10 normal values (nv); GGT, x12 nv; ALP, x4 nv). The HCV RNA level was 11 million IU/mL. The absolute lymphocyte counts were deeply reduced indicating an impaired B and T lymphocytes immune-reconstitution (CD3+ lymphocytes, 450 cells/μι; CD4+ cells, 63; CD8+ cells, 427; and B lymphocytes, 0). In April 2011, biochemical exams showed a further increase in cholestasis (GGT, x18 nv; bilirubin, x16 nv; ALP, x6 nv). Serum protein electrophoresis shows a very low IgG Immunoglobulin dosage (390 mg/dL) and HCV RNA level increased to 147 million IU/mL. Abdominal ultrasonography showed slight ascites. The patient underwent a liver biopsy, which revealed acute hepatitis characterized by hepatocytic degeneration, cholestasis, neutrophilic infiltration at the parenchymal-portal interface and pericellular fibrosis (Figures 1A and 1B), indicating a diagnosis of cholestatic hepatitis C. After two months, for a futher increase of cholestasis a second liver biopsy was repeated. Histological examination revealed the presence of fibrous septa connecting adjacent portal tracts and porto-central tracts, pericellular fibrosis, cholestasis and mild lymphocytic and neutrophilic infiltration of the portal tract (grading 11, staging 4 according to the Ishak classification) (Figures 1C and 1D). Treatment with pegylated interferon-alfa 2a (Pegasys Hoffmann-La Roche Welwyn Garden City UK) was started at a dose of 180 μg/week along with ribavirin (Rebetol Merck Sharp Dohme Hertfordshire UK) 1,000 mg/day per os. After 1 month of treatment, HCV RNA level decreased to 1,810 IU/mL (reduction of 4.96 logw), but there was only a slight reduction in cholestasis (GGT, x15 nv; bilirubin, x10 nv; ALP, x10 nv). The patient continued the antiviral treatment, and tested negative for HCV RNA 40 days after starting antiviral treatment. Antiviral treatment was stopped after 6 months. An end of treatment virological response (EVR), normalization of the ALT, AST and total bilirubin levels (1.0 mg/100 mL) was observed. Liver cholestasis was markedly reduced (GGT, x3 nv; ALP, x2 nv). A slight increase of lymphocytes count appeared starting from 3 months post treatment. A persistence of a low IgG plasmatic level (450 mg/dL) was noted. The patient achieved a sustained virologic response (SVR) 6 months after the end of therapy, and progressive normalization of the cholestasis occurred. In March 2013 the patient presented a recurrence of DLBCL and computed tomography showed multiple lesions located in the right and left pulmonary lobe as recurrence of DLBCL. HCVRNA was negative in serum. The patient died two months later despite a chemotherapy was instituted. All the modifications of biochemical, virological and immunologic parameters over the time are reported in the figure 2.
Liver histological findings. A. The first liver biopsy, performed at presentation, revealed balloon degeneration of the hepatocytes (black arrow) and cholestasis. A component of inflammatory infiltrates was present at the parenchymal-portal interface (original magnification, x20; hematoxylin and eosin stain). B. Pericellular fibrosis (white arrow) and ballooning of hepatocytes (black arrow) were also observed in the first biopsy specimen (original magnification, x20; Van Gieson stain). C. A second liver biopsy performed 7 weeks after the first showed diffuse inflammatory infiltrates and cholestasis (original magnification, x20; hematoxylin and eosin stain). D More extensive fibrosis with fibrous expansion of the portal tracts, and portal-to-portal and portal-to-central bridging were also seen (original magnification, x10; Van Gieson stain).
A. Trends in ALT, GGT during and after chemotherapy. Values of ALT, GGT and ALP are showed during and after antiviral therapy. B. Trend in HCVRNA during and after chemotherapy and during and after antiviral therapy. C. Trend in total bilirubin during and after chemotherapy and during and after antiviral therapy. D. Trend in CD4+, CD8+ and lymphocyte B during and after chemotherapy and during and after antiviral therapy.
This is the first reported case of cholestatic hepatitis C after rituximab-containing chemotherapy in a patient with a DLBCL describes in the literature. Cholestatic hepatitis C was first described after LT and can lead to accelerated allograft injury and fibrosis because of high levels of HCV replication and the ongoing suppression of the host immune response to viral antigens.2,3 The HCV load results to be very high in this condition, usually > 6 log10 and is associated to an important cholestasis.7 Hepatic injury is thought to be the result of direct intracellular viral cytoxicity as a consequence of very high HCV load in patients with severe immunosuppression. Immunosuppression increases HCV entry into hepatocytes via upregulation of the entry receptor occludin combined with spread of the virus from cell to cell. In addition, owing to the strong immunosuppression, there is a failure to mount a detectable HCV-specific T-cell response. In this condition, high levels of interleukin (IL)-8 have been observed because of direct viral stimulation of IL-8 production and potential impairment of antiviral type I interferon response.8 This form of hepatitis due to excess HCV antigens is poorly responsive to antiviral treatment and has a high mortality. The pathogenetic mechanism described above can theoretically occur in all patients with strong immunosuppression. Several studies have shown that the frequency of HCV infection is high in patients with some subtypes of B-cell NHLs.9 Liver dysfunction during chemotherapy containing rituximab in HCV-positive patients with NHL has been described in several studies.4,6,10 Liver damage in HCV infected patients during and post chemotherapy containing Rituximab could be due essentially to three different pathogenetic mechanisms. The first mechanism is due to chemotherapy toxicity. In fact it is demonstrated that some chemotherapeutic agents, including cyclophosphamide, vincristine, doxorubicin and carboplatin, have been associated with hepatotoxicity.11–13 Furthermore rituximab-immunomediated phenomena are also a possible causative mechanisms of hepatic damage.14,15 Rituximab itself might induce liver damage, and HCV infection is a risk factor for the development of rituximab-induced liver damage. In the iatrogenic damage high HCV RNA levels, with respect to the baseline levels, are noted. A second pathogenetic mechanism that can occours at a variable time of 1-200 days after discontinuation of chemotherapy containing Rituximab is related to an “immunological rebound” with restoration of immune function and increased inflammatory activity in the liver, resulting in rapid destruction of HCVinfected hepatocytes and liver injury.16 In this last case, variable levels of HCV RNA have also been observed, but without a strict correlation between HCV RNA levels and grade of liver damage.6,17 Some Authors reported very low levels of HCV RNA in patients with severe hepatitis C several months after chemotherapy as a result of a hyperergic immune system activation.17,18 Merli, et al. demonstrated that in 255 HCV-positive NHL patients who were treated with rituximab-containing chemotherapy, increase in HCV RNA level did not always show a clear direct correlation with severe hepatoxicity.19 A third pathogenetic mechanism is due to direct HCV intracellular viral cytoxicity as a consequence of very high HCV load and this condition is so called “cholestatic hepatitis C”. Cholestatic hepatitis is frequent in the first phase of immunosuppression in which frequently high values of plasmatic HCVRNA levels are present for a profound immunosuppression.7 In the case herein described cholestatic hepatitis was disclosed after 1-2 months from the end of chemotherapy. In our patient persistence of immunosuppression (low B and T cell count) after the discontinuation of chemotherapy containing rituximab could have resulted in liver damage via a similar pathogenetic mechanism as that described in LT patients.1,7 The elevated intrahepatic HCV load observed in our patient was a reflection of the failure of immune reconstitution, and it induced intracellular oxidative stress and mitochondrial dysfunction, which in turn were responsible for the cholestatic damage. But we believe that this was not the only mechanism that realized a cholestatic hepatitis in our patient. In the past medical history the patient reported several episodes of sinusitis and bronchitis and two episodes of pneumonia due to Klebsiella pneumoniae. Furthermore this patient had a persistence low dosage of circulating IgG immunoglobulin present before the advent of NHL. We cannot clearly demonstrate but can only suspect that the patient could have had a common variable immune deficiency (CVID). This condition is characterized by several episodes of recurrent infections involving ears, nasal sinuses, bronchi and lungs and is associated to very low levels of immunoglobulins. In CVID extranodal B cell type lymphomas are a frequent complication and are more common in subjects in the fourth to seventh decade of life.20,21 We can speculate that this condition associated with HCV infection was probably the predispose factors for NHL. In our patient, antiviral therapy with pegylated interferon plus ribavirin led to an SVR and to a resolution of cholestatic hepatitis. New second generation direct acting antiviral agents (DAAs) are more effective antiviral regimens and could in future improve the poor prognosis of this dreadful complication, particularly, in patients infected with “diffficult-to-treat” HCV genotypes.22 Furthermore a unique case report describes a rapid HCV negativization and complete remission of a marginal lymphoma during II generation DAAs treatment without the use of interferon and chemotherapy.23 Currently no clinical studies have analyzed the role of second generation DAA’s in DLBCL patients treated with chemotherapy containing Rituximab. We believe that Interferon free treatments could be administered in these patients for different reasons: high viral clearance, no demonstrated interaction with chemotherapy, low collateral effects respect to interferon treatment, preventive with respect to HCV viral reactivation post chemotherapy. It was extensively demonstrated that the reduction of dose intensity due to therapy toxicity has been already recognized as an adverse prognostic factor for DLCBL. To date no dosage or modification of chemotherapy regimens are expected in patients with DLBCL and HCV infection.24 In our case, despite a resolution of HCV infection, recurrence of lymphoma was noted. This could have been promoted by the presence of a condition of persistent immunodeficiency (CVID). To our knowledge, this is the first case report worldwide of cholestatic hepatitis C in a patient with DLBCL treated with rituximab-containing chemotherapy.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
CVID: common variable immune deficiency.
- •
CVP-R: cyclophosphamide, vincristine, prednisone plus rituximab.
- •
GGT: gammaglutamyl transpeptidase.
- •
HCV: hepatitis C virus.
- •
IL8: interleukin 8.
- •
NHL: non-Hodgkin lymphoma.