Trimethoprim-Sulfomethoxazole (TMP-SMX) related hepatotoxicity and associated severe systemic reaction are not frequent and documented only in case reports. We report a case of a 30-year-old man, who underwent a 15-day therapy with TMP-SMX for urinary tract infection and two weeks later developed acute cholestatic hepatitis, fever and a skin rash followed by severe systemic reaction. He was admitted in Intensive Care unit and with supportive therapy and prednisolone administration, he showed subsequent improvement over a period of few days. He had fully recovered months later. All tests for other causes of liver disease were negative and his liver biopsy showed evidence of drug-induced hepatic injury.
Drug-induced hepatotoxicity accounts for more than 50 percent of the cases of acute liver failure in the United States, while more than 75 percent of idiosyncratic drug reactions result in liver transplantation or death.1
Trimethoprim-Sulfamethoxazole (TMP-SMX) is one of the most commonly used antibiotics, with a broad spectrum of indications and although its use has been reduced in recent years, it is still the antibiotic of choice for Pneumonocystis carinii pneumonia and toxoplasmosma prophylaxis in HIV-positive patients.2
Adverse reactions to TMP-SMX occur in 8% of hospitalized patients, usually involving the skin and the gastrointestinal tract, although only 3% of these adverse reactions represent sulfonamide-hypersensitivity reaction.3,4
Severe systemic reactions, as result of TMP-SMX hypersensitivity, are even less frequent and are mostly documented as case reports.5
Case reportA 30-year-old Caucasian male was admitted to our department with a four-day history of fatigue, high fever (40° C) without rigors, skin rash of the trunk and extremities, discoloration of urine, mild right upper quadrant pain and vomiting.
He was normally fit and well, up until one month earlier, when he had suffered a urinary tract infection. For that, he was prescribed Trimethoprime-Sulfomethoxazole (800+160 mg) for 15 days. He had never used TMP-SMX before. Accidentally though, he had taken double dose of the medication (1920 mg instead of 960 mg) twice daily.
He had no risk factors for chronic liver disease and no relevant family history.
On physical examination, he had mild jaundice and an erythematous, maculopapular rash over the trunk and extremities. The abdominal examination revealed mild hepatomegaly, with smooth liver edge and tenderness, as well as splenomegaly. There were no signs of chronic liver disease and no enlarged lymph nodes.
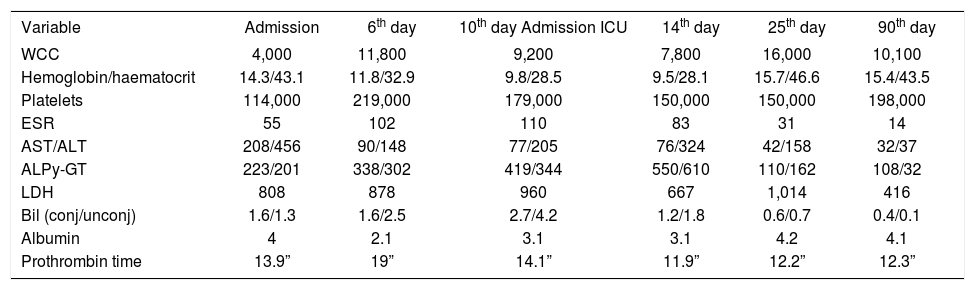
Laboratory testing on admission revealed abnormal liver biochemistry with elevated aminotransferases (AST 208 U/mL; range 0-40, ALT 456 U/mL; range 0-40), elevated cholestatic enzymes (ALP 223 U/L; range 40-140, γ-GT 201U/L; range 0-40), elevated bilirubin (direct 1.6 mg/dL-indirect 1.3 mg/dL), prolonged prothrombin time (PT 13.9 sec), raised LDH (808 U/L; range 50-280) as well as mild leucopenia (WCC 3, 900), thrombocytopenia (PLT 114, 000) and raised ESR (55 mm/1st h). Albumin level was normal; he had slightly raised creatinine (1.6 mg/dL; range 0.44-1.43) but normal urea nitrogen. Electrolytes were normal (Table I). Bilirubin levels in urine were also raised. Radiographs of the chest and abdomen were normal. The abdominal ultrasound revealed homogenous hepatomegaly with normal intrahepatic bile ducts and splenomegaly (17 cm), while the heart ECHO was normal.
Blood chemical findings.
| Variable | Admission | 6th day | 10th day Admission ICU | 14th day | 25th day | 90th day |
|---|---|---|---|---|---|---|
| WCC | 4,000 | 11,800 | 9,200 | 7,800 | 16,000 | 10,100 |
| Hemoglobin/haematocrit | 14.3/43.1 | 11.8/32.9 | 9.8/28.5 | 9.5/28.1 | 15.7/46.6 | 15.4/43.5 |
| Platelets | 114,000 | 219,000 | 179,000 | 150,000 | 150,000 | 198,000 |
| ESR | 55 | 102 | 110 | 83 | 31 | 14 |
| AST/ALT | 208/456 | 90/148 | 77/205 | 76/324 | 42/158 | 32/37 |
| ALPy-GT | 223/201 | 338/302 | 419/344 | 550/610 | 110/162 | 108/32 |
| LDH | 808 | 878 | 960 | 667 | 1,014 | 416 |
| Bil (conj/unconj) | 1.6/1.3 | 1.6/2.5 | 2.7/4.2 | 1.2/1.8 | 0.6/0.7 | 0.4/0.1 |
| Albumin | 4 | 2.1 | 3.1 | 3.1 | 4.2 | 4.1 |
| Prothrombin time | 13.9” | 19” | 14.1” | 11.9” | 12.2” | 12.3” |
The initial working diagnosis was that of acute (druginduced, viral or autoimmune) cholestatic hepatitis with differential from other causes of liver disease with unusual presentation and systemic diseases causing hepatic involvement.
The patient was treated with intravenous (IV) fluids and antipyretic agents. Full viral screen (HBV, HCV, HAV, Eb-stein-Barr Virus, Herpes Simple Virus, Cytomegalovirus, HIV), autoantibodies (ANA, AMA, SMA, ANCA, Anti-LKM1) and a detailed septic screen were negative. Despite the initial supportive therapy though, the patient deteriorated further. He developed constant pyrexia, productive cough, his oxygen saturation fall (92%) and pitting oedema of the legs appeared. His chest X-ray showed an elevation of the right hemidiaphragm and pulmonary infiltrates of the mid-and lower pulmonary fields.
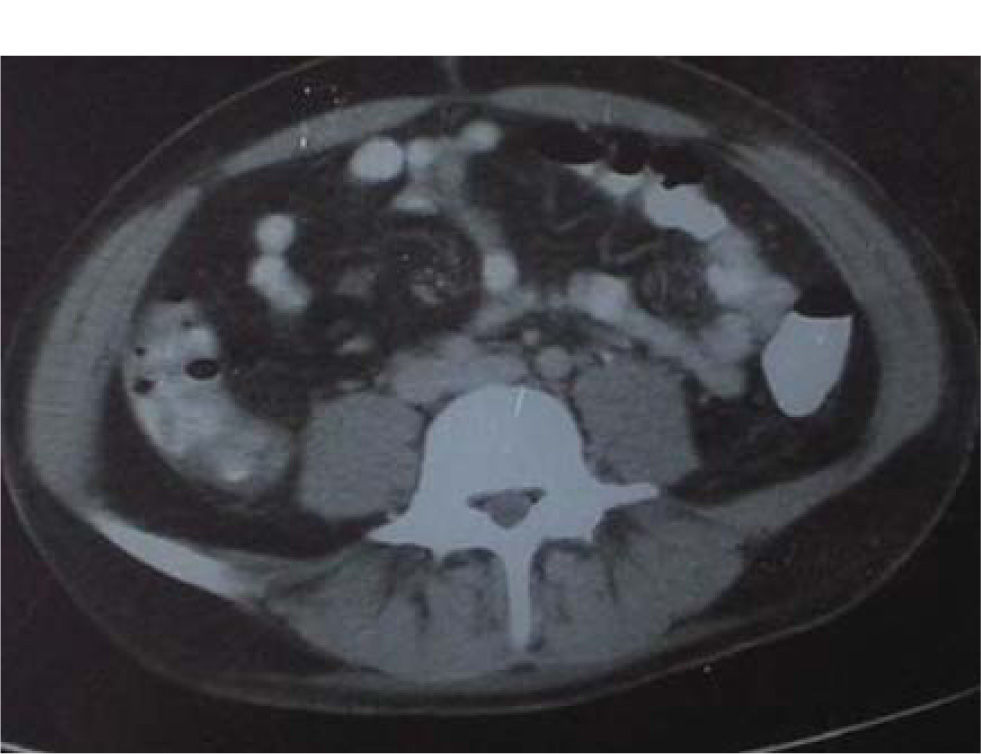
A CT scan of his abdomen showed enlargement of the spleen and liver without focal lesions, presence of ascitic fluid and thickening –in places-of the bowel wall (Figure 1).
As the patient continued to deteriorate, prednisolone 100 mg/day was instituted after an initial IV dose of 1 g. Eventually, the patient was intubated and transferred to the Intensive Care Unit with SIRS (SOFA score 14), hypotension (BP 80/40 mmHg) and metabolic alkalosis. The skin rash had started to exfoliate.
On day 12 of his in-hospital stay, the fever started to subside and the cardiorespiratory function to improve. Two days later, he was transferred back to the ward. He remained pyrexial, but his cardiorespiratory function was normal and the biochemistry was improving (Table I). Prednisolone was lowered to a dose of 75 mg/day IV.
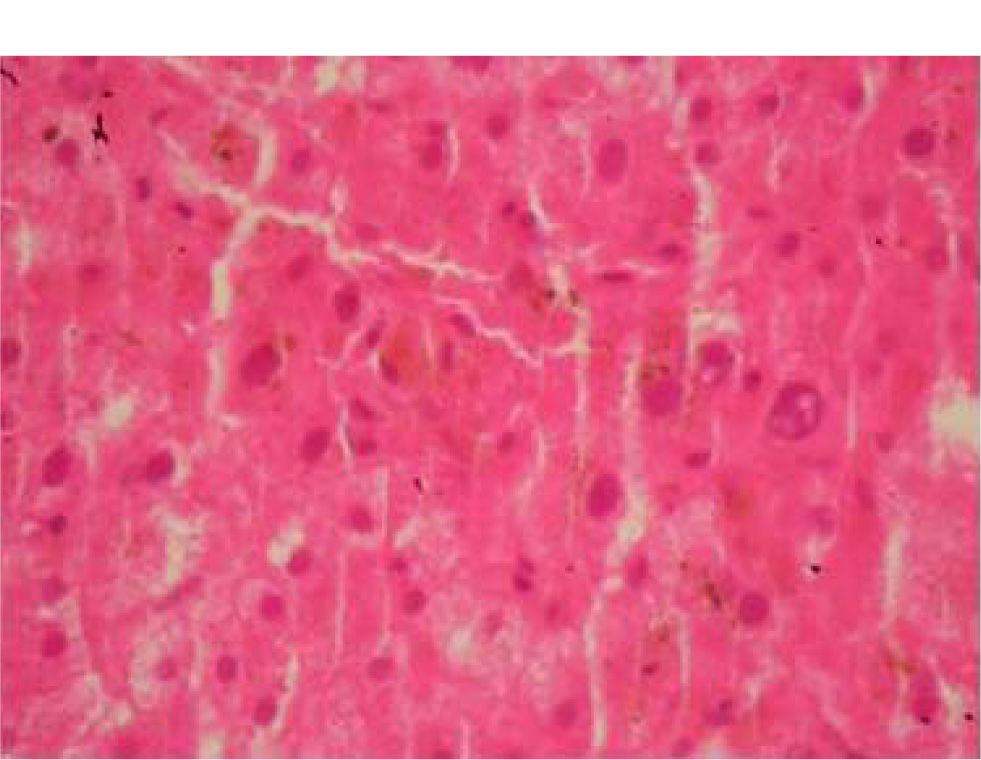
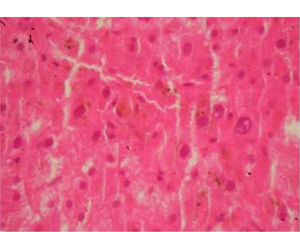
At this stage, a liver biopsy was performed and examination of the specimen revealed infiltration of the portal triads with lymphocytes and centrilobular cholestasis (Figure 2), features rather suggestive of a drug-induced cholestatic hepatitis. The patient was eventually discharged after a while and over the last six months he remains asymptomatic with normal biochemical profile.
DiscussionMost drugs cause liver injury infrequently and this vary in severity from mild and transient increase of serum aminotransferase levels to fulminant hepatic failure. These reactions are either idiosyncratic, occurring usually 5-90 days from the initial ingestion or dose-dependent (acetaminophen).6
We presented a patient who, fifteen days after TMP-SMX therapy developed cholestatic hepatitis, along with a hypersensitivity reaction (fever, skin rash) by a severe systemic reaction (cardiorespiratory failure, hepatic failure with prolonged prothrombin time, hypo-albuminaemia and thrombocytopenia) which progressively resolved with supportive therapy and corticosteroids.
Trimethoprim-sulfamethoxazole is associated with many adverse effects including hepatic injury, nausea, arthritis, electrolyte abnormalities, anaphylaxis, cutaneous eruptions, leucopenia, thrombocytopenia and hemolytic anemia.7 The hepatic injury can be either cholestatic or mixed,8,9 associated with pruritus and jaundice and may even cause bile ducts to vanish (vanishing bile duct syndrome).10,11 TMP-SMX is also associated with a hypersensitivity syndrome characterized by fever, a generalized maculopapular rash, eosinophilia and occasionally toxicity to other organs (hypotension, pulmonary infiltrates) which develops 7 to 14 days after initiating therapy and usually resolves with supportive therapy.12 Even cases of fulminant hepatic failure related to TMP-SMX use were described.13-15
Several mechanisms have been proposed for the sulfonamide hypersensitivity however; it still remains poorly understood. SMX forms an intermediate product (SMX-NHOH) after oxidation within the hepatic cytochrome P-450. Further oxidation of SMX-NHOH yields the nitroso-compound, SMX-NO. Both of them act as haptens to host proteins or exert a direct cytotoxic effect to lymphocytes and other immune cells.16 The “danger hypothesis” suggests that a stimulator signal, such as an infection, can result in activation of the immune system leading to immune responses to a drug otherwise well tolerated.17
We suggest that this case represents an idiosyncratic reaction to TMP-SMX which manifested as cholestatic hepatitis along with hypersensitivity syndrome and followed by severe systemic reaction. Accidental self-administration of higher than usual dose had played major role in the severity of symptoms. The patient’s improvement (with no clinical, laboratory or radiographic findings of permanent liver injury), points towards an immune-mediated response to TMP-SMX which resulted in a temporary but severe clinical condition. This case also shows that, the use of corticosteroids in drug-induced hepatic injuries, had resulted in either faster improvement or a decrease in the inflammatory and immune processes.