Little is known about primary biliary cholangitis (PBC) in non-whites. The purpose of this study was to evaluate clinical features and outcomes of PBC in a highly admixed population.
Material and methodsThe Brazilian Cholestasis Study Group multicentre database was reviewed to assess demographics, clinical features and treatment outcomes of Brazilian patients with PBC.
Results562 patients (95% females, mean age 51 ± 11 years) with PBC were included. Concurrent autoimmune diseases and overlap with autoimmune hepatitis (AIH) occurred, respectively, in 18.9% and 14%. After a mean follow-up was 6.2 ± 5.3 years, 32% had cirrhosis, 7% underwent liver transplantation and 3% died of liver-related causes. 96% were treated with ursodeoxycholic acid (UDCA) and 12% required add-on therapy with fibrates, either bezafibrate, fenofibrate or ciprofibrate. Response to UDCA and to UDCA/fibrates therapy varied from 39%-67% and 42-61%, respectively, according to different validated criteria. Advanced histological stages and non-adherence to treatment were associated with primary non-response to UDCA, while lower baseline alkaline phosphatase (ALP) and aspartate aminotransferase (AST) levels correlated with better responses to both UDCA and UDCA/fibrates.
Conclusions: Clinical features of PBC in highly admixed Brazilians were similar to those reported in Caucasians and Asians, but with inferior rates of overlap syndrome with AIH. Response to UDCA was lower than expected and inversely associated with histological stage and baseline AST and ALP levels. Most of patients benefited from add-on fibrates, including ciprofibrate. A huge heterogeneity in response to UDCA therapy according to available international criteria was observed and reinforces the need of global standardization.
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease of unknown etiology which has a variable rate of progression towards cirrhosis and end-stage liver disease. PBC predominantly affects middle-aged women and is usually associated with several extra-hepatic autoimmune disorders. The incidence and prevalence rates have been estimated at 0.9 to 5.8 and 1.91 to 40.2 per 100,000 people in Europe, North America, Asia and Australia [1]. The disease is thought to be rare in Latin America and Africa. Previous studies have shown that PBC may have a more aggressive course in Hispanics and African Americans, suggesting that genetic background may modulate biological processes related to the clinical expression and progression of the disease [2,3].
Little is known about PBC affecting subjects with heterogeneous genetic backgrounds [4]. Brazil has a population of a highly admixed origin, with varying proportions of Amerindian, African, and European genetic ancestries, shaped by local historical interactions with migrants brought by the slave trade, European settlement, and indigenous populations [5]. In order to gather data on clinical features and treatment outcomes of PBC in the country, the Brazilian Society of Hepatology sponsored a multicenter cooperative consortium named as Brazilian Cholestasis Study Group comprised by investigators from both academic and community-based institutions who manage and treat patients with cholestasis, including PBC. This paper presents the results of real-world data concerning the clinical phenotype and treatment outcomes of PBC in Brazil.
2Material and methods2.1Study populationThe study population included adult (≥ 18 years old) patients who were diagnosed with PBC between January 1st, 1992 and December 31st, 2019 in 28 different hepatology centers throughout the country. All procedures were conducted in accordance with the ethical standards of the Helsinki Declaration and the study was approved by the Federal University of Minas Gerais Ethics Committee Board (CAAE 98627218.6.1001.5149). The diagnosis of PBC was considered if the patient fulfilled at least two of the following diagnostic criteria as recommended by the American Association for the Study of Liver Disease guidelines: (i) positive serology for anti-mitochondrial antibodies (AMA); (ii) persistent increase of the serum alkaline phosphatase (ALP) levels; and (iii) liver histology compatible with PBC [6]. Autoimmune hepatitis (AIH) and PBC overlap syndrome was considered if patient satisfied the Paris criteria [7]. Patients in whom the diagnosis could not be confirmed or who had another etiology of liver disease were excluded. AMA status was assessed by indirect immunofluorescence. All AMA positive patients had titers ≥ 1:40. Liver histology specimens were available for all patients with AMA negative-PBC.
2.2Data collectionEach researcher was asked to identify all PBC patients that have been followed in their Liver Center at the time of the survey, without any selection or exclusion whatsoever, and to fill-in a standardized database provided by the Brazilian Cholestasis Study Group, which was reviewed by two independent investigators (GGLC, CAC). Clinical data obtained from medical records included: sex, age at diagnosis, year of diagnosis, year of first symptoms or first biochemical changes, last date of follow-up, liver histology (fibrosis was staged according to the Ludwig system), extra-hepatic manifestations, AMA status, serum liver biochemistry, ursodeoxycholic acid (UDCA) and/or fibrate treatment, liver decompensation (ascites, variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis), transplantation and death. Data on liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT) and ALP, were collected at diagnosis and 6 months to 5 years after treatment, when available. Biochemical results were normalized by upper limit of normality (ULN) of each laboratory in order to homogenize the interpretation of the data. The considered standardized daily dose of UDCA for PBC treatment was 13–15 mg/kg of body weight [6]. The response to treatment either to UDCA or fibrates was analyzed according to the Barcelona, Paris I and II, Toronto and POISE trial criteria [8–12]. Treatment compliance was determined at the physician's discretion as regular, irregular or sporadic use. The threshold for regular use was defined as taking ≥ 80% of pills, whereas a rate < 80% indicated irregular adherence. Sporadic use was considered in patients who randomly took the medication [13]. The duration of follow-up was defined as the interval between the diagnosis and the last visit or the date of liver transplantation or death. Baseline ALP and AST levels therapy were compared to treatment response adjusted for compliance, according to those aforementioned criteria.
2.3Statistical analysisStatistical analysis was performed using SPSS 25.0 software (IBM, USA). Continuous variables distribution was assessed by Shapiro-Wilk test, and those with Gaussian distribution were expressed as mean and standard deviation, or as median and interquartile range (IQR) if skewed distribution. Categorical variables were expressed as absolute number and percentage. Univariate analysis was performed using chi-square or Fisher exact test, as appropriate, for categorical variables. Continuous variables were analyzed by the Student t-test or Mann-Whitney U-test, according to the distribution. Pairwise deletion was applied to missing data. P-values < 0.05 were considered statistically significant.
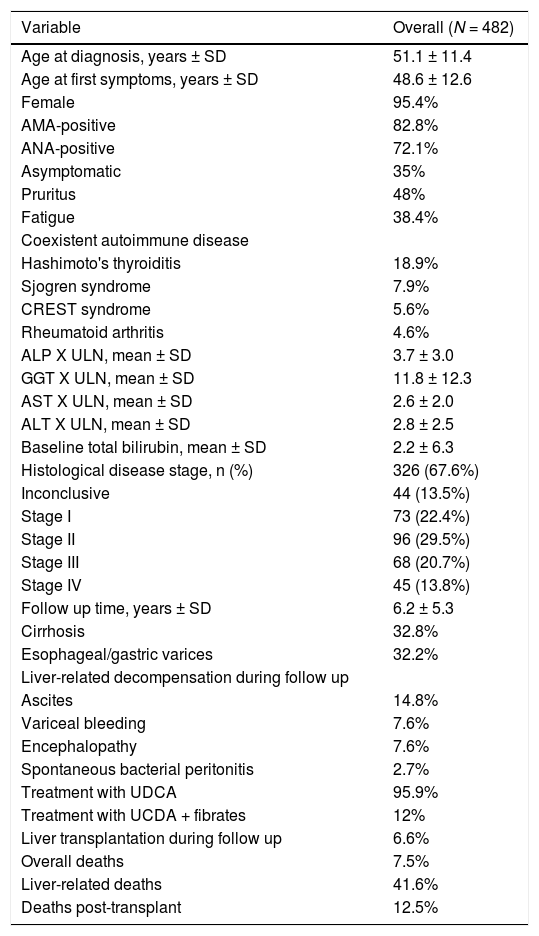
3Results3.1Patient CharacteristicsA total of 562 patients with PBC were included in this study. Clinical and laboratory features of the study population are summarized in Table 1. Eighty patients (14.2%) had overlap syndrome with AIH according to Paris criteria and were excluded from outcome analyses. The majority of subjects were middle-aged women (95%; mean age 51 ± 11 years) with classical symptoms of pruritus and/or fatigue, which were observed in 65% of the patients. Mean time to diagnosis, considering time between first symptoms or biochemical changes and definite diagnosis, was 2.5 years. The prevalence of AMA was 82.8%, while anti-nuclear antibody was found in 72.1%. Liver pathology at diagnosis was available for 326 patients (67.6%). One third of them had PBC stage III or IV according to Ludwig classification [14]. After a mean follow-up of 6.2 ± 5.3 years, 32% of the subjects had clinical, laboratory or imaging evidence of cirrhosis. Esophageal varices were present in one third of the patients. Requirement for liver transplantation and liver-related deaths were observed in 6.6% and 3.2% of the patients, respectively. Hepatocarcinoma was diagnosed in 1.9% of the subjects. Hashimoto's thyroiditis (18.9%) was the most common coexistent autoimmune disease, though Sjögren syndrome (7.9%), CREST syndrome (5.6%) and rheumatoid arthritis (4.6%) were also frequently reported.
Brazilian primary biliary cholangitis cohort characteristics
Data are expressed as absolute number/data available (percentage). SD: standard deviation; n = number; AMA: anti-mitochondrial antibodies; ANA: anti-nuclear antibody; ALP: alkaline phosphatase; GGT: gamma-glutamyltransferase; AST: aspartate aminotransferase; ALT: alanine aminotransferase; UDCA: ursodeoxycholic acid.
Ninety six per cent of the patients were treated with UDCA in a mean dose of 13 ± 2.6 mg/kg/day. The remaining subjects were UDCA intolerant due to bloating and diarrhea. Treatment compliance was considered regular, irregular and sporadic, respectively, in 81%, 18% and 1% of them. None of the patients required drug discontinuation due to side effects. Fifty-nine patients (12%) were treated with fibrates in association with UDCA due to an inadequate response to UDCA, 14% of them had cirrhosis. Overall, 47.5%, 47.5% and 5% of the patients used ciprofibrate (mean dose = 95.4 ± 14.6 mg/day), bezafibrate (mean dose = 358 ± 82 mg/day) and fenofibrate (mean dose = 167.7 ± 40.8 mg/day), respectively. Mean time between starting UDCA and adding fibrates was 19.7 months. Data on adverse events or temporary discontinuation were not available for fibrates analysis. Forty-five patients (92%) reported regular treatment compliance to UDCA and fibrates therapies.
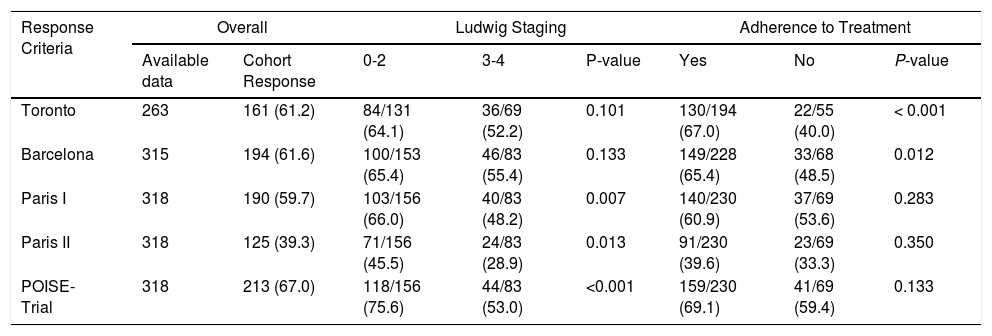
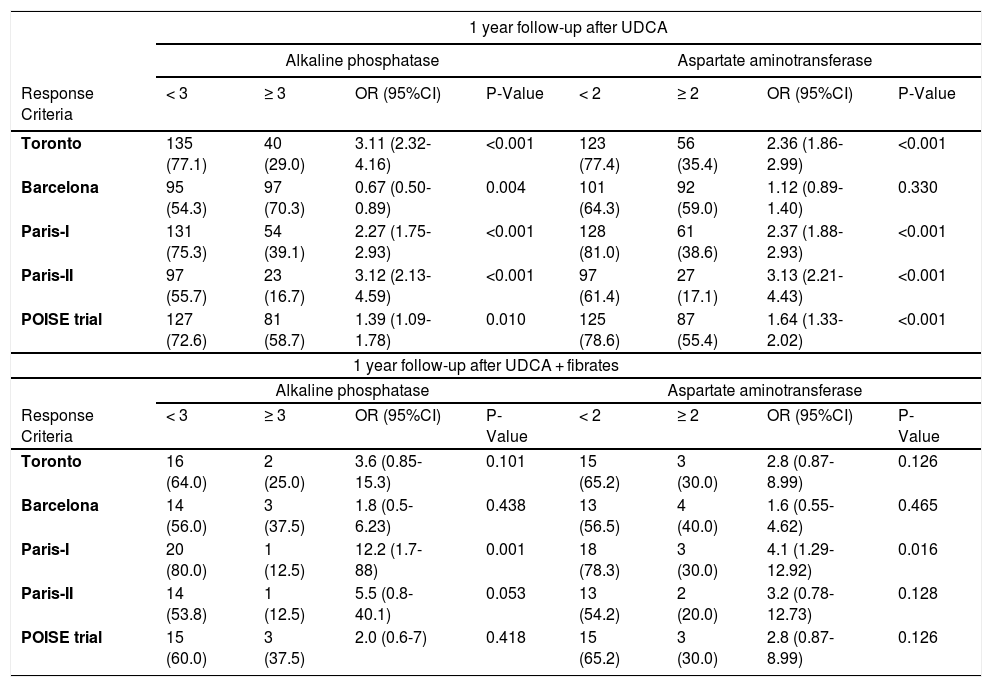
3.2Response to UDCA treatmentResponse to UDCA treatment was assessed in all patients based on the availability of laboratory parameters and varied from 39 to 67% according to the different criteria (Table 2). Lower rates of response were observed using Paris II criteria. Advanced histological stages were associated with non-response according to Paris I, II and POISE criteria, while non-adherence to treatment was associated with treatment failure using the Toronto and Barcelona criteria (Table 2). We also evaluated efficacy of UDCA by different criteria stratified by pre-treatment ALP levels (≥ 3 X ULN vs < 3 X ULN) at baseline. When using all available criteria, patients with baseline ALP < 3 X ULN presented a statistically better response to UDCA when compared to their counterparts with higher levels of ALP before treatment (Table 3). Similarly, we observed a strong inverse relationship between AST ≥ 2 X ULN and response to treatment to all but the Barcelona criteria (Table 3).
Response to ursodeoxycholic acid treatment according to different criteria
Data are expressed as absolute number/available data (percentage). Chi-square test was performed.
Comparison of response to ursodeoxycholic acid and fibrates by different criteria stratified by alkaline phosfatase and aspartate aminotransferase/upper limit of normality ratios at pre-treatment baseline.
OR, odds ratio; CI, confidence interval; UCDA, ursodeoxycholic acid. Data are expressed as absolute number (percentage). Chi-square test was performed.
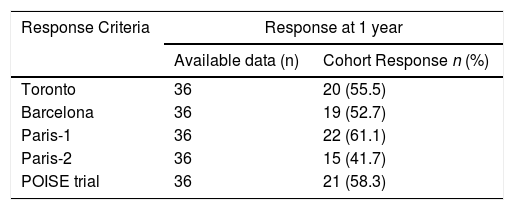
Overall rates of response to combined therapy of UDCA and fibrates according to different criteria are reported in Table 4 and varied from 42–61%. Response rates did not differ according to stage of fibrosis, adherence to treatment and at 2 years follow-up (data not shown). Response rates were higher in those subjects with baseline AST < 2 X ULN and baseline ALP < 3 X ULN, but the difference was statistically significant only with the use of Paris I criteria (Table 3). Differently, ALT level (≥ 2 X ULN or < 2 X ULN) at baseline was not able to predict response to fibrates by any criteria (data not shown).
4DiscussionLittle is known about PBC in non-white subjects who have been underrepresented historically in clinical trials. The Brazilian population presents a highly diverse ancestry with varying percentages of Caucasoid, Negroid and Amerindian ancestries not amenable to race self-classification or further characterization based on expression of morphological traits such as skin color [15]. In this study, we addressed clinical features, disease progression and real-world response to treatment in a unique highly admixed multicenter cohort of Brazil. Previous studies assessing non-whites from North America revealed that African Americans and Hispanics may have a more aggressive disease compared with their Caucasian counterparts [2,3]. Lower 1-year survival and higher in-hospital mortality was also reported in African Americans in at least two other studies, possibly due to delayed diagnosis and lower access to treatment with UDCA [16–19]. In addition, features of overlap syndrome of AIH and PBC, higher rates of decompensation over time and lower response to UDCA were also more likely to be observed in Hispanics [3]. In the present study, clinical features of PBC, including demographics and disease presentation, were similar to those previously reported worldwide, except for a higher female-to-male ratio (21:1), longer time to diagnosis, remarkable frequency of advanced PBC and lower AMA reactivity [1,6,20]. These findings may be explained by an under-recognition of the disease in Brazil, a variability of AMA testing practices throughout the country, as well as by referral bias or even a different disease phenotype in Latin America.
Treatment with UDCA has been shown to improve serum liver biochemistry, delay histological progression and increase transplant-free survival [1,9,21,22]. Response to UDCA in our cohort was lower than expected using different international criteria, but, as previously reported, response to therapy correlated to baseline AST and ALP levels and histological stage of PBC [23]. Interestingly, the proportion of patients with symptomatic disease at diagnosis was higher than observed in other cohorts [24]. Most of our patients had baseline symptoms of fatigue and/or pruritus, which were previously associated with a more aggressive disease, lower response to UDCA, as well as higher progression to cirrhosis and its complications [24]. We also demonstrated that adherence to treatment is a crucial point to be analyzed in real life studies since it directly impacts biochemical response at all times. Furthermore, the previously reported overexpression of overlap syndrome of AIH and PBC in US non-whites was not observed in our population with a highly heterogeneous genetic background [3]. Nonetheless, the prevalence of AIH-PBC overlap syndrome was similar to the rates described in the literature (10-15%) [25]. Finally, a low rate of hepatocellular carcinoma (HCC) was observed, further confirming that there is a significant difference in HCC incidence between viral and autoimmune liver diseases [26].
Since obeticholic acid is not approved in Brazil as second-line treatment of PBC, most of the subjects with non-response to UDCA were switched to off-label add-on therapy with fibrates. Previous studies have described significant decrease in ALP associated with bezafibrate and fenofibrate treatment [27–31]. To the best of our knowledge, this is the first study to report ciprofibrate use in PBC. The majority of our patients achieved biochemical response independently of the fibrate chosen. This finding is extremely relevant, since whereas most commercially available fibrates, including fenofibrate and ciprofibrate, specifically activate PPARα, bezafibrate is a pan PPAR-agonist, activating all three PPAR subtypes (α, γ and δ) at comparable doses [32]. In this way, clinical implications of this differential PPAR selectivity in PBC remain undefined and seems not to be relevant. Furthermore, the addition of fibrates appeared to induce the strongest beneficial effect in patients with baseline ALP < 3 X ULN and AST < 2 X ULN, probably reflecting lower severity of biliary and hepatic injury. Interestingly, this difference is not observed when using obeticholic acid as an add-on treatment, which induced durable improvements in markers of hepatic injury and cholestasis, regardless of baseline ALP and total bilirubin levels, in a subanalysis of the POISE trial [33].
This is the first study to describe PBC characteristics and response to treatment in an unique large and genetically diverse Brazilian cohort. However, some limitations which are inherent of retrospective study designs should be taken into consideration. The sample size was limited by missing data, especially for the analyses involving biochemical response. In order to optimize sample representativeness during analysis, we performed pairwise deletion of missing data. In addition, heterogeneity in the availability of AMA and its different assessment methods between participating centers may also have influenced the rates of AMA positivity and the proportion of patients with liver histology evaluation. Furthermore, data on adverse events or temporary discontinuation were not available for safety evaluation of fibrates.
5ConclusionsIn summary, clinical and laboratory features of PBC in Brazilians are similar to those previously reported in Caucasian and Asian subjects, with lower rates of AIH-PBC overlap syndrome. Response to UDCA is lower than expected and is inversely associated with histological stage, adherence to treatment and baseline AST and ALP levels. On the other hand, most of the patients unresponsive to UDCA benefited from an add-on therapy with different marketed fibrates in Brazil, including bezafibrate, fenofibrate and the less studied ciprofibrate. Finally, our work sheds light on the huge heterogeneity between prognostic scores and the need for a global standardization.
CRediT authorship contribution statementGuilherme Grossi Lopes Cançado: Conceptualization, Visualization, Data curation, Formal analysis, Writing – original draft. Michelle Harriz Braga: Visualization, Data curation, Writing – original draft. Maria Lúcia Gomes Ferraz: Visualization, Data curation, Writing – original draft. Cristiane Alves Villela-Nogueira: Visualization, Data curation, Writing – original draft. Debora Raquel Benedita Terrabuio: Visualization, Data curation, Writing – original draft. Eduardo Luiz Rachid Cançado: Visualization, Data curation, Writing – original draft. Mateus Jorge Nardelli: Visualization, Data curation, Writing – original draft. Luciana Costa Faria: Visualization, Data curation, Writing – original draft, Formal analysis. Nathalia Mota de Faria Gomes: Visualization, Data curation, Writing – original draft. Elze Maria Gomes de Oliveira: Visualization, Data curation, Writing – original draft. Vivian Rotman: Visualization, Data curation, Writing – original draft. Maria Beatriz de Oliveira: Visualization, Data curation, Writing – original draft. Simone Muniz Carvalho Fernandes da Cunha: Visualization, Data curation, Writing – original draft. Daniel Ferraz de Campos Mazo: Visualization, Data curation, Writing – original draft. Liliana Sampaio Costa Mendes: Visualization, Data curation, Writing – original draft. Claudia Alexandra Pontes Ivantes: Visualization, Data curation, Writing – original draft. Liana Codes: Visualization, Data curation, Writing – original draft. Valéria Ferreira de Almeida e Borges: . Fabio Heleno de Lima Pace: Visualization, Data curation, Writing – original draft. Mario Guimarães Pessoa: Visualization, Data curation, Writing – original draft. Izabelle Venturini Signorelli: Visualization, Data curation, Writing – original draft. Gabriela Perdomo Coral: Visualization, Data curation, Writing – original draft. Paulo Lisboa Bittencourt: Conceptualization, Visualization, Data curation, Writing – original draft, Writing – review & editing. Cynthia Levy: Visualization, Data curation, Writing – original draft. Cláudia Alves Couto: Writing – review & editing, Conceptualization, Visualization, Data curation, Writing – original draft, Writing – review & editing.
This work was supported by Brazilian Society of Hepatology and Instituto Brasileiro do Fígado – IBRAFIG.