When a patient is suspected of chronic hepatitis C virus (HCV) infection, the following examinations should be conducted.
I. An epidemiological study should be conducted to determine if the patient has an increased risk of HCV infection. Recommendations for routine HCV testing have been published by the CDC (Center for Disease Control), AASLD (American Association for the Study of Liver Diseases), and NIH (National Institute of Health).
CDCInstances in which routine tests for HCV are recommended:
- •
prior use of illegal drugs,
- •
receipt of coagulation factors before 1987,
- •
receipt of transfusion/organs before July 1992,
- •
chronic hemodialysis, and
- •
evidence of liver disease.
- •
health personnel, emergency and general workers after injury or exposure to the blood of HCV-positive persons,
- •
children of HCV-positive mothers.
- •
for health personnel, emergency and co-workers,
- •
during pregnancy,
- •
when there has been no sexual contact with HCV-positive persons, or
- •
for the general population.
- •
recipients of transplanted tissue,
- •
drug addicts who do not inject themselves and use intranasal cocaine,
- •
people who have a history of tattoos and body piercing,
- •
people with a history of sexually transmitted disease or multiple sexual partners, or
- •
sexual partners of long-term HCV-positive persons.
The recommendations of the NIH are the same as those of the CDC except for the following stipulations.
- •
Persons who received blood transfusions or organs before 1990 should undergo testing for HCV (CDC uses 1992 as the cutoff year).
- •
Individuals with multiple sexual partners, couples, those who have had contact with HIV-positive patients, and those who have shared instruments involved in the nasal aspiration of cocaine should be screened for HCV.
The AASLD recommends HCV screening for the following groups:
- •
persons who have administered drugs intravenously, even if this occurred only once,
- •
HIV-positive persons who received coagulation factors before 1987, underwent hemodialysis or exhibited inexplicable increases in transaminase activity,
- •
people who received transfusions or transplanted organs before 1992,
- •
children of HCV-positive mothers,
- •
health personnel, emergency workers, and co-workers who have been injured or exposed to blood mucus of HCV-positive persons, and
- •
sexual partners of persons infected with HCV.
We use the foregoing tests to determine if a patient is at risk of contracting hepatitis C.1-3
II. Fatigue is the most frequent symptom mentioned in clinical histories of HCV-positive patients. Other less common symptoms are nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. The symptoms are not disabling, and their association with liver disease is not obvious. The symptoms of chronic HCV infection do not reflect the activity of the disease. Symptoms commonly appear once cirrhosis has developed.
III.At the physical examination, we look for evidence of chronic liver disease (jaundice, telangiectasia, gynecomastia, hepatomegalia, splenomegalia, collateral circulation, ascites, or edemas).
IV.In our laboratory biochemical study, we test for the presence of liver necrosis (concentrations of SGOT (Serum glutamic oxaloacetic transaminase) and SGPT (Serum glutamic pyruvic transaminase)) and cholestasis (concentrations of FA, bilirubin, and GGTP (gammaglutamylotranspeptydase)) and evaluate liver function (prothrombin time, albumin concentrations).
One third of patients with chronic virus C infection have normal transaminase (SGOT and SGPT) levels. Only 25% have more than double the normal level of ALT (Alanine aminotransferase), and transaminase levels greater than 10 times normal are rare.
There is a poor correlation between transaminase concentrations and liver histology. The AST (aspartate aminotransferase) /ALT ratio may be useful for predicting the presence of cirrhosis. An AST/ALT ratio ≥ 1 has 100% specificity and 53% sensitivity for cirrhosis, and a positive predictive value of 100% and a negative predictive value of 81%.4
V.If evidence of chronic liver disease is detected during the clinical, physical or biochemical examinations, we carry out an etiological study as follows.
1. HCV ELISA (Enzyme Linked Inmunoabsorvent Assay) 2 or 3. 2. HBs Ag (Hepatitis B surface antigens) and anti HBs for virus B. 3. Ceruloplasmin for Wilson and Alpha 1 antitrypsin for lack of alpha 1 antitrypsin. 4. Cells LE (Lupus erythematosus), antinuclear antibodies and anti-DNA for autoimmune chronic liver disease. 5. Antimitochrondial antibodies for primary gallstone cirrhosis. 6. Indexes of iron and ferritin for hemochromatosis 7. Lipids for nonalcoholic steatohepatitis.
VI.If there is evidence of chronic liver disease, we conduct cabinet studies using ultrasound of the superior abdomen, computer axial tomography or liver scintigraphy with colloidal technetium sulfur to detect possible redistribution of the disease to the spleen, bone marrow, and lungs. A liver biopsy can also be done when there is evidence of chronic liver disease, even if it is not mandatory. Of people that are HCV-positive according to the HCV ELISAs results and have normal transaminase levels, 27% have normal liver histology or slightly altered liver histology, 54% exhibit chronic hepatitis with portal inflammation with without [sic] periportal extension, and 19% have chronic hepatitis with a moderate level of inflammatory activity but no fibrosis. The remaining had cirrhosis.5 The histopathological characteristics of hepatitis C are steatosis, gallstone lymphoid aggregates, and damage to bile ducts.6
VII.The HCV ELISA is easy to use and relatively cheap. HCV ELISAs tests are used at present. HCV ELISA 2 detects the following recombinant antigens: C22, C33, C100. The assay has a sensitivity of 95% and a predictive value of 88%–95% in high-risk populations and 50%–61% in low risk populations. This technique reduced the seroconversion period to 10 weeks after exposure. The HCV ELISA 3 detects the nonstructural antigen, NS5, which provides the best sensitivity in the highrisk group and the best specificity in the donor population. The seroconversion period is reduced to two or three weeks.7-9
VIII.If the results of the HCV ELISAs 2 or 3 are positive, we conduct a confirmatory test, of which there are two types.
- 1.
Recombinant immunoblot assay (RIBA). The RIBA 2 and RIBA 3 tests are used for screening of blood bank donors for whom the ELISA HCV gives positive results.
- •
2. Molecular tests. HCV RNA can be measured qualitatively or quantitatively as follows.
•PCR or reverse transcription. These are quantitative methods. Genetic material is extracted, subjected to reverse transcription, and quantified by PCR after denaturalization, hybridization, and extension. There are two types of PCR: endpoint PCR, which gives a result after 30 or 40 cycles, and real-time PCR, in which extension and detection occur at the same time.
•Branched DNA tests (bDNA). This is a technically easy and flexible test that has less probability of contamination than PCR but has low sensitivity. Of samples that are PCR-positive for HCV RNA, 10%–30% are bDNAnegative for HCV RNA.
These molecular tests assist the physician to determine whether the virus is present. There most popular commercial quantitative PCR kits are:
- 1.
Quantiplex HCV RNA v 2.0 (Bayer Diagnostics, Puteaux, France),
- 2.
Cobas Amplicor HCV Monitor assay (COBAS v 2.0 Roche Diagnostics Systems,
- 3.
LCX ABBOTT end point), and
- 4.
ABI-Prism p-7000 ABBOTT (real time) If a test is used, it must be carried out to monitor the response to treatment.
- 1.
There is a high level of variability in the PCR between laboratories. Recently, the American College of Pathologists initiated a quality control scheme for the PCR.
- 2.
There is no accepted standard for this test. The results of one test are not comparable with those of another.
- 3.
Many factors can affect the sensitivity and specificity of the test.
- 4.
Other factors that contribute to variability are the storage conditions of samples, the design of amplification primers, variability of biochemical reactions, DNA product contamination, and the efficiency of systems for postamplification detection.
It is important to mention that in immunosuppressed cases of chronic HCV disease, the results of the HCV ELISA may be negative when those of PCR tests are positive.10-12
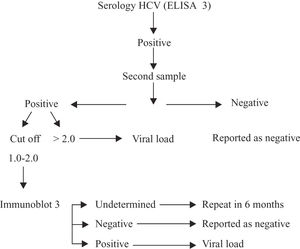
In Costa Rica, we use the following flowchart to detect HCV in blood collected by voluntary blood banks.
Conclusions- 1.
In cases of suspected chronic HCV infection, obtain the medical history and conduct clinical, physical, and laboratory examinations.
- 2.
Request an HCV ELISA 2 or 3 and a quantitative PCR if positive.
- 3.
Study other associated causes of chronic liver disease.
- 4.
Conduct a complete cabinet study with US(ultransonography), CAT (Computerized Axial Tomography), and hepatosplenic scans.
- 5.
A biopsy is not mandatory.
- 6.
In cases of chronic cryptogenic liver disease, request a viral PCR test even if the HCV ELISA is negative.
- 7.
For HCV screening in blood banks, use the RIBA-3 test for confirmation if the HCV ELISA is positive. If the RIBA-3 test is inconclusiveinconsistent, repeat after six months and proceed with a PCR if positive.
1. Should screening for HCV be recommended for patients who had blood transfusions prior to the establishment of mandatory screening tests by blood banks?
Each country should recommend that HCV ELISA 2 or 3 tests be carried out for individuals with histories of transfusion with blood or blood derivatives prior to compliance with mandatory regulations for HCV testing of blood bank samples.
The quality of evidence for this recommendation was given a rating of 2
What screening test should be used for patients suspected of chronic HCV infection?
Determination of the presence of anti-HCV antibodies using second-or third-generation ELISA tests is recommended for any person with suspected HCV infection. The use of the RIBA test to confirm infections is valid in Latin American countries until the new tests become available.
The quality of evidence for this recommendation was given a rating of 2
Which is the best test for the diagnosis of acute HCV infection?
The recommended test for diagnosis of acute HCV infection is qualitative or quantitative detection of HCV RNA by real-time PCR.
The quality of evidence for this recommendation was given a rating of 2
What are the diagnostic criteria for chronic hepatitis C?
The diagnostic criteria established by the consensus panel are:
- •
positive for Anti-HCV antibody,
- •
HCV RNA detected, and
- •
persistent increase of alanine aminotransferase (ALT) aminotransferase levels for more than six months or normal transaminase levels with detectable HCV RNA.
Liver biopsy is not a necessary criterion. The quality of evidence for this recommendation was given a rating of 2
Is it necessary to carry out autoimmune tests for patients who are chronically infected with HCV?
The panel did not reach consensus. However, autoimmune tests should be considered in certain cases.
It is necessary to detect HCV RNA and determine the HCV genotype to determine the nature of therapy? Both are strictly necessary.
The quality of evidence for this recommendation was given a rating of 1
Is it acceptable to initiate and monitor treatment using the core antigen test in the event of a lack of the technical or financial resources required to conduct molecular tests?
There was no consensus. However, there is evidence that the core antigen assay may be used to monitor treatment.