FibroTouch® has shown efficacy in staging hepatic fibrosis in patients with chronic viral hepatitis B, but its performance in assessing liver steatosis and fibrosis in metabolic dysfunction-associated steatotic liver disease (MASLD) patients remains understudied. We aimed to evaluate the diagnostic performance of FibroTouch® in assessing steatosis and fibrosis in the MASLD population.
Materials and MethodsLiver stiffness measurements and steatosis were assessed using FibroTouch® and FibroScan®, with FibroScan® as the reference standard. Pearson's correlation test evaluated correlations, and kappa statistics determined agreement between the two methods. Optimal cut-off values of FibroTouch® for predicting hepatic steatosis and fibrosis stages were determined through ROC curve analysis with the Youden index method.
ResultsStrong correlations were observed between FibroTouch® UAP and FibroScan® CAP (rho=0.74) and LSM values (rho=0.87) (p < 0.001 for both) in a total of 380 patients. The mean CAP value for the entire cohort was 285 ± 51 dB/m, and the median LSM for the cohort was 5 .3kPa. The optimal FibroTouch® UAP cutoffs were 229 dB/m for S0 vs. S1, 267 dB/m for S1 vs. S2, and 294 dB/m for S2 vs. S3. For FibroTouch® LSM, the optimal cutoffs were 6.0 kPa for F0-F1 vs. F2, 7.9 kPa for F2 vs. F3, and 10.6 kPa for F3 vs. F4. Moreover, FibroTouch® effectively assessed hepatic steatosis and fibrosis in patients with different BMIs.
ConclusionsFibroTouch® proved valuable in assessing hepatic steatosis and liver fibrosis staging in MASLD patients, enhancing its applicability in various clinical settings as a suitable and convenient option for MASLD patients.
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), previously termed non-alcoholic fatty liver disease (NAFLD) [1], is rapidly becoming a prevalent cause of chronic liver conditions worldwide. Its incidence is anticipated to escalate significantly in the coming years [2]. The presence of fibrosis stands out as the primary feature linked to an elevated risk of developing cirrhosis or hepatocellular carcinoma (HCC), causing increased morbidity and mortality [3]. Consequently, timely detection and effective management of liver fibrosis in MASLD assume paramount significance [4]. This enables prompt intensive lifestyle modifications and utilization of potential therapeutic options to halt disease progression and improve long-term outcomes for MASLD patients.
Liver biopsy traditionally considered the ‘gold standard’ for evaluating liver fibrosis over the last few decades, has several limitations [5]. These include its invasive nature, potential complications, patient discomfort, and susceptibility to sampling errors [5–7]. Vibration Controlled Transient Elastography (VCTE) by FibroScan® was developed to measure liver stiffness in patients with different chronic liver diseases [8]. VCTE offers the advantages of being painless, rapid (usually less than 5 min), and easy to perform at the bedside or in the outpatient clinic, with a key feature of FibroScan® being its simultaneous assessment of hepatic fat content through a parameter known as controlled attenuation parameter (CAP) [9]. The primary limitation of FibroScan® arises from its impracticality in certain patient populations, such as those who are morbidly obese or possess substantial chest wall fat, which hinders the procedure, diminishes efficiency, and potentially compromises success rates due to inherent technical constraints [10].
Introduced in 2013, FibroTouch® (Wuxi Hisky Medical Technology Co., Ltd., Wuxi, China) is a newer tool featuring an integrated two-dimensional image guiding system that enables precise positioning and depth measurement by automatically adjusting the dynamic probes according to the thickness of subcutaneous fat, thereby simplifying the procedure and improving accuracy [11]. FibroTouch® assesses hepatic steatosis and fibrosis by generating a mechanical pulse that induces an elastic shear wave in the liver tissue; the velocity of this wave, tracked by ultrasound, determines liver stiffness, while the ultrasound attenuation parameter (UAP) estimates fat content [12]. Compared to the widely validated FibroScan®, which operates on a similar principle, FibroTouch® offers shorter examination times, image-guided positioning, and a larger sampling volume, improving measurement accuracy and providing a comprehensive assessment of liver tissue [12,13]. However, FibroTouch® has a limited evidence base and faces challenges in distinguishing closely related stages of steatosis and fibrosis. Additionally, while FibroScan® employs multiple probes tailored for different body types, enhancing accuracy in obese patients, FibroTouch® uses a single probe frequency [14]. Prior studies have demonstrated promising results generated by the FibroTouch® with a successful measurement rate of 100 %, offering greater convenience and wider applicability for fibrosis assessment [15–17].
In a study involving 190 patients with chronic liver disease, not specifically focusing on NAFLD, who underwent liver biopsy and liver stiffness measurement (LSM) using FibroTouch®, a significant correlation (r = 0.804, p = 0.000) was observed between LSM and histological fibrosis [18]. In a prospective study conducted on 435 chronic liver disease patients, including 15 (3.4 %) patients with NAFLD, FibroTouch® demonstrated an area under the receiver operating curve (AUROC) similar to FibroScan® for diagnosing significant fibrosis, severe fibrosis, or cirrhosis. Additionally, a significant correlation was found between FibroTouch® and FibroScan® for liver stiffness (r = 0.85, p < 0.001) [19]. Another comparative analysis involving 1621 patients, not exclusively comprising NAFLD cases, to assess the efficiency of FibroScan® and FibroTouch® in LSM and fat quantification, the LSM and fat quantification results obtained from FibroTouch® showed a significant correlation with those from FibroScan® (r = 0.645 and p = 0.620) [20]. However, studies evaluating the performance of FibroTouch® in assessing liver steatosis and fibrosis in MASLD patients are still limited. We hypothesized that FibroTouch® has comparable diagnostic accuracy to FibroScan® for these purposes. Therefore, this study aimed to assess the diagnostic accuracy of FibroTouch® for staging steatosis and fibrosis in MASLD patients, using FibroScan® as the reference standard.
2Materials and Methods2.1Study designThis study was conducted as a single-center, cross-sectional study at the Center of Excellence for Innovation and Endoscopy in Gastrointestinal Oncology, Division of Gastroenterology, Department of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand.
2.2ParticipantsBetween January 2022 and August 2023, we enrolled 380 patients with a diagnosis of MASLD who visited the Hepatology Clinic at King Chulalongkorn Memorial Hospital, identified through ICD code K76.0. The diagnosis of MASLD relied on ultrasound evidence of fatty liver or CAP scores of ≥233 dB/m from FibroScan® results. Exclusions encompassed patients with other chronic liver diseases such as chronic viral hepatitis B or C infection, autoimmune hepatitis, pregnant individuals, and those with ascites. Fig. 1 is the research flow chart. We calculated the sample size based on a sensitivity (P) of 0.507, an acceptable error (d) of 10 %, and an alpha level of 0.05, which corresponds to a critical Z-value of 1.96. Using the formula n = Zα2xPx(1−P)d2, we determined a total sample size of 380 patients [21]. We selected a sensitivity (P) of 0.507 based on the research by Zhu et al. in 2021, which referenced the diagnostic performances of UAP values for patients with NAFLD at hepatic steatosis stage 3 (S3) [22]. This calculation ensured adequate statistical power to evaluate the diagnostic accuracy of FibroTouch® in comparison to FibroScan®.
2.3Assessment of patientsBoth FibroScan® and FibroTouch® measurements were performed during the same visit after a 2-h fast. Two experienced operators, each with over 1000 examinations in their respective techniques, conducted the measurements independently and were blinded to each other's results.
2.4FibroScan® CAP and LSMFibroScan® (FibroScan® 502 touch, Echosens, Paris, France) initially utilized the M probe at depths of 25–65 mm. If unsuccessful, the XL probe at depths of 35–75 mm was employed, primarily for patients with obesity or thick subcutaneous fat. Measurements were taken on the right liver lobe through the 9–11th intercostal spaces, with patients lying supine and their right arm maximally abducted (Fig. 2a). The objective is to obtain ten acceptable measurements, defined as a successful LS measurement, with a maximum of 20 attempts allowed. Each measurement is categorized as “very reliable” (IQR/M ≤ 0.1), “reliable” (0.1 < IQR/M ≤ 0.3 or IQR/M > 0.3 with LS median <7.1 kPa), or “poorly reliable” (IQR/M > 0.3 with LS median ≥7.1 kPa) based on the criteria proposed by Boursier [23].
(a): Illustration of the FibroScan® Measurement Technique, (b): Illustration of the FibroTouch® Measurement Technique.
The FibroTouch® measurement (FibroTouch-FT100, Wuxi Hisky Med, China) was also performed by a blinded operator. Measurements targeted the right liver lobe through the 7–9th intercostal spaces, with patients in a supine position (Fig. 2b). Successful measurement criteria included a success rate exceeding 60 % and an IQR/median liver stiffness ratio below 30 % [13].
2.6Data collectionBaseline demographics, including age, gender, body mass index (BMI), and underlying diabetes mellitus were collected for all participants. Patients were classified into three BMI groups based on the Asian Pacific Association for the Study of the Liver (APASL) guidelines: normal weight (BMI ≤ 22.9 kg/m2), overweight (BMI 23.0–24.9 kg/m2), and obese (BMI ≥ 25.0 kg/m2) [24]. Laboratory results for albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) were abstracted from the electronic medical record. Liver stiffness values (in kilopascal, kPa), along with CAP and UAP values (in dB/m) measured by FibroScan® and FibroTouch®, were documented. Non-invasive hepatic steatosis and fibrosis markers, including fibrosis-4 index (FIB-4), aminotransferase to platelet ratio index (APRI), AST/ALT ratio, steatosis-associated fibrosis estimator (SAFE) score and hepatic steatosis index (HSI) were also computed using the available parameters.
FibroScan® staging for steatosis and fibrosis used established cut-off values [25,26]. For steatosis: S0 is no significant steatosis, with less than 5 % of hepatocytes containing fat (CAP ≤ 233 dB/m); S1 is mild steatosis, with 5–33 % of hepatocytes affected (CAP ≤ 268 dB/m); S2 is moderate steatosis, with 34–66 % of hepatocytes affected (CAP ≤ 300 dB/m); S3 is severe steatosis, with more than 66 % of hepatocytes affected (CAP ≥ 301 dB/m [25,27]. For fibrosis: F0 is no fibrosis; F1 is mild fibrosis, i.e., portal fibrosis (VCTE ≤ 5.7 kPa); F2 is moderate fibrosis, i.e., portal fibrosis with few septa (VCTE ≤ 7.0 kPa); F3 is advanced fibrosis, i.e., portal fibrosis with numerous septa (VCTE ≤ 10.2 kPa); F4 is cirrhosis (VCTE ≥ 10.3 kPa) [26,28].
2.7Statistical analysisStatistical analyses were conducted using STATA 17.0 (StataCorp LLC, College Station, TX). The results are presented as mean ± standard deviation (SD) for normally distributed data and as median (interquartile range: IQR) for non-normally distributed data. Categorical variables were described using frequency (percentage). FibroScan® was used as a reference standard. If the data were normally distributed, differences between steatosis and fibrosis stages were compared using one-way ANOVA. If the data were not normally distributed, the Kruskal-Wallis test was employed for comparison. Additionally, correlations were assessed using Pearson's correlation test. Bland–Altman analysis evaluated the agreement between the FibroTouch® and FibroScan®, with the limits of the agreement (mean ± 1.96 times the SD of the differences) determined. We used the One-vs-One strategy for multi-class classification, i.e., S0 vs. S1, S1 vs. S2, S2 vs. S3 for steatosis stages, and F0-F1 vs. F2, F2 vs. F3, F3 vs. F4 for fibrosis stages, to determine optimal FibroTouch® cutoffs for predicting hepatic steatosis and fibrosis stages, targeting at least 90 % sensitivity or specificity. For the ROC curve analysis, cut-off values optimizing the Youden index were also calculated for comparison of LSM values, as well as UAP and CAP values of FibroScan® and FibroTouch®. We calculated the Cohen's Kappa (κ) statistic to assess agreement between FibroScan® and FibroTouch® measurements for liver stiffness and steatosis stages, interpreting κ values as follows: <0: no agreement, 0.01–0.20: slight, 0.21–0.40: fair, 0.41–0.60: moderate, 0.61–0.80: substantial, and 0.81–1.00: almost perfect. Weighted Kappa was also used to account for the ordinal nature of stages, providing a more detailed measure of agreement based on the severity of discrepancies. We applied the UAP and LSM cutoffs of FibroTouch® from previous studies and company recommendations to our cohort to assess and compare their diagnostic performance with our proposed FibroTouch® cutoffs. Qu et al. [29] used cutoffs of S1 (≥244 dB/m), S2 (≥269 dB/m), and S3 (≥296 dB/m) for steatosis, and F0-F1 (≤5.2 kPa), F2 (≤6.0 kPa), F3 (≤10.9 kPa), and F4 (≥11.0 kPa) for fibrosis. Yu et al. [30] determined cutoffs of S1 (≥278 dB/m), S2 (≥305 dB/m), and S3 (≥307 dB/m) for steatosis, and F2 (≥12.8 kPa), F3 (≥13.8 kPa), and F4 (≥20.1 kPa) for fibrosis. Zhu et al. [22] used S1 (≥295 dB/m), S2 (≥314 dB/m), and S3 (≥324 dB/m) for steatosis. Hisky Medical recommended S0 (<240 dB/m), S1 (240–264 dB/m), S2 (265–294 dB/m), and S3 (≥295 dB/m) for steatosis, and F0-F1 (<8.0 kPa), F2 (8.0–11.0 kPa), F3 (11.0–15.0 kPa), and F4 (≥15.0 kPa) for fibrosis. A p-value of <0.05 was considered statistically significant.
2.8Ethical statementAll patient records were thoroughly anonymized and de-identified before analysis. Written informed consent was obtained from all participants by investigators. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No. 384/64).
3Results3.1Patients characteristicsA total of 380 participants were enrolled in the study, with a mean age of 55.8 ± 13.3 years and 160 (42.1 %) males. The median (IQR) BMI for the cohort was 26.2 (23.5–29.1) kg/m2. The distribution of BMI categories was as follows: normal weight – 19.7 %, overweight – 20.6 %, and obese – 59.7 %. Diabetes is present in 101 (26.6 %) of patients (Table 1).
Patient characteristics.
| Characteristics | Total patients (n = 380) |
|---|---|
| Age (years) (mean ± SD) | 55.8 ± 13.3 |
| Male (n, %) | 160, 42.1 |
| Diabetes Mellitus (n, %) | 101, 26.6 |
| Body Mass Index (BMI) (kg/m2) [median (IQR)] | 26.2 (23.5–29.1) |
| Subgroup based on BMI | |
| 75, 19.7 |
| 78, 20.6 |
| 227, 59.7 |
| Platelets count (x 109/L) | 295 (226–309) |
| Albumin (g/dL) [median (IQR)] | 4.4 (4.2–4.5) |
| Alanine Transaminase (ALT) (IU/L) [median (IQR)] | 29 (20–47) |
| Aspartate Transferase (AST) (IU/L) [median (IQR)] | 24 (20–32) |
| Alkaline Phosphatase (ALP) (IU/L) [median (IQR)] | 69 (57–84) |
| Gamma-glutamyl Transferase (GGT) (IU/L) [median (IQR)] | 37 (27–66) |
| Fibrosis-4 Index (FIB-4) [median (IQR)] | 1.03 (0.70–1.40) |
| AST to Platelet Ratio Index (APRI) [median (IQR)] | 0.0024 (0.0020–0.0046) |
| AST/ALT ratio [median (IQR)] | 0.85 (0.69–1.08) |
| Steatosis-associated Fibrosis Estimator (SAFE) Score (mean ± SD) | 33.75 ± 82.14 |
| Hepatic Steatosis Index (HSI) [median (IQR)] | 38.10 (33.62–43.07) |
IQR; Interquartile Range, SD; Standard Deviation.
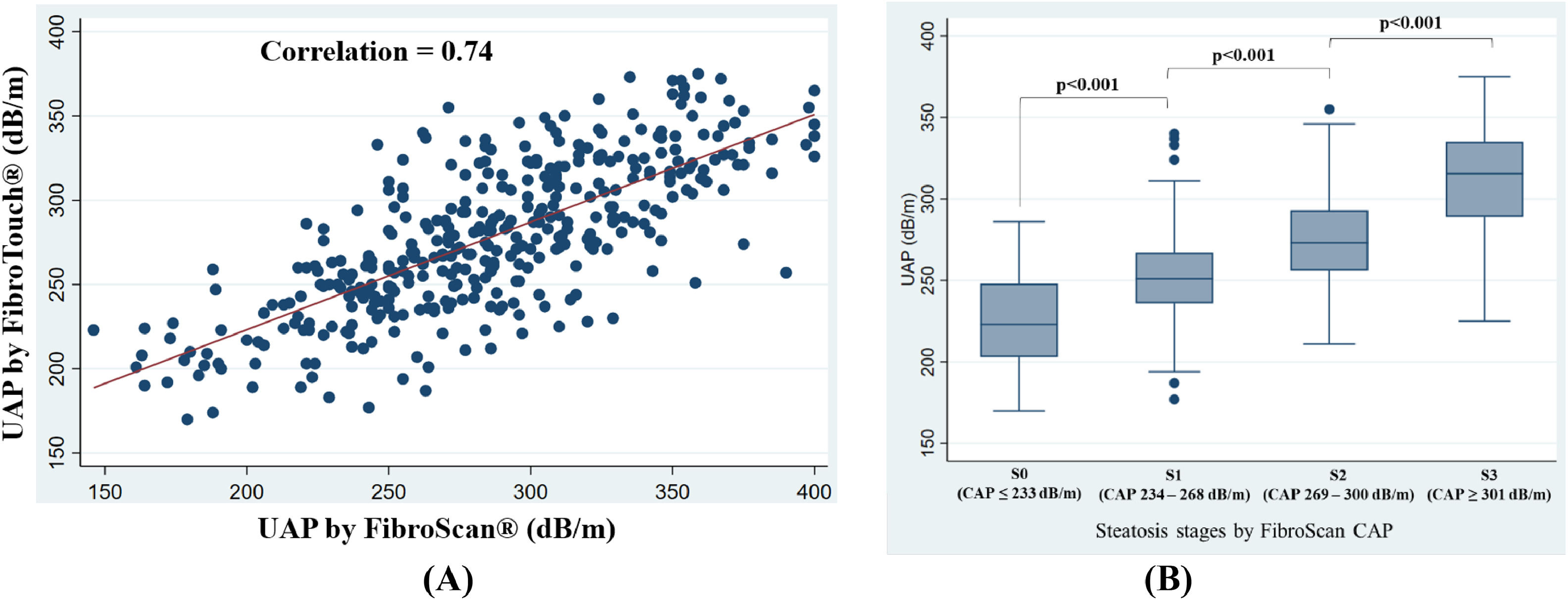
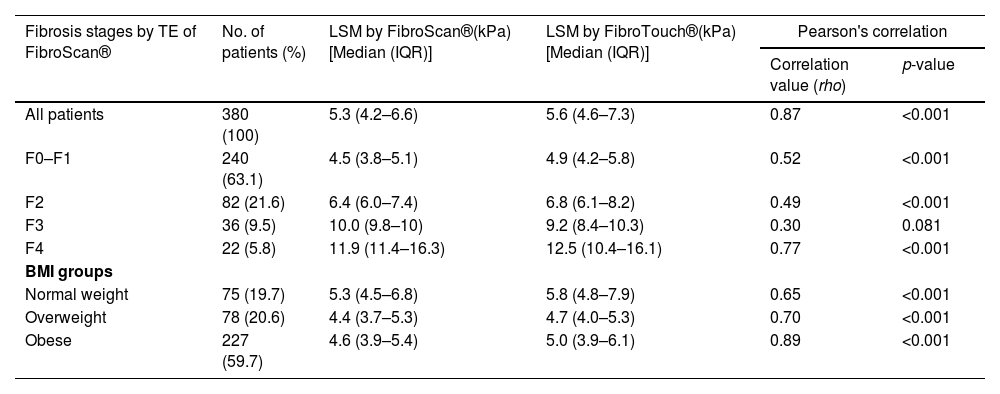
The entire cohort had a mean CAP of 285±51 dB/m. Hepatic steatosis prevalence was: S0 (15.0 %, CAP 205±23 dB/m), S1 (21.3 %, CAP 251±9 dB/m), S2 (25.0 %, CAP 284±9 dB/m), and S3 (38.7 %, CAP 337±26 dB/m) (Table 2). The cohort had a median LSM of 5.3 kPa (IQR 4.2–6.6). Liver fibrosis stages were: F0-F1 (63.1 %, LSM 4.5 kPa, IQR 3.8–5.1), F2 (21.6 %, LSM 6.4 kPa, IQR 6.0–7.4), F3 (9.5 %, LSM 10.0 kPa, IQR 9.8–10.0), and F4 (5.8 %, LSM 11.9 kPa, IQR 11.4–16.3) (Table 3).
CAP measurements of FibroScan® vs. UAP measurements FibroTouch® and correlation in subgroups of different stages of hepatic steatosis and in subgroups of BMI.
| Steatosis stages by CAP of FibroScan® | No. of patients (%) | FibroScan® CAP(dB/m)(Mean ± SD) | FibroTouch® UAP(dB/m)(Mean ± SD) | Pearson's correlation | |
|---|---|---|---|---|---|
| Correlation value (rho) | p-value | ||||
| All patients | 380 (100) | 285 ± 51 | 278 ± 44 | 0.74 | <0.001 |
| S0 | 57 (15.0) | 205 ± 23 | 225 ± 27 | 0.50 | <0.001 |
| S1 | 81 (21.3) | 251 ± 9 | 255 ± 32 | 0.22 | 0.050 |
| S2 | 95 (25.0) | 284 ± 9 | 277 ± 31 | 0.13 | 0.211 |
| S3 | 147 (38.7) | 337 ± 26 | 312 ± 33 | 0.42 | <0.001 |
| BMI groups | |||||
| Normal weight | 75 (19.7) | 241 ± 50 | 241 ± 37 | 0.74 | <0.001 |
| Overweight | 78 (20.6) | 272 ± 40 | 265 ± 34 | 0.69 | <0.001 |
| Obese | 227 (59.7) | 304 ± 45 | 294 ± 41 | 0.63 | <0.001 |
CAP; Controlled Attenuation Parameter, SD; Standard Deviation, UAP; Ultrasound Attenuation Parameter, BMI; Body Mass Index.
LSM measurements of FibroScan® vs. FibroTouch® and correlation in subgroups of different fibrosis stages and in subgroups of BMI.
| Fibrosis stages by TE of FibroScan® | No. of patients (%) | LSM by FibroScan®(kPa)[Median (IQR)] | LSM by FibroTouch®(kPa)[Median (IQR)] | Pearson's correlation | |
|---|---|---|---|---|---|
| Correlation value (rho) | p-value | ||||
| All patients | 380 (100) | 5.3 (4.2–6.6) | 5.6 (4.6–7.3) | 0.87 | <0.001 |
| F0–F1 | 240 (63.1) | 4.5 (3.8–5.1) | 4.9 (4.2–5.8) | 0.52 | <0.001 |
| F2 | 82 (21.6) | 6.4 (6.0–7.4) | 6.8 (6.1–8.2) | 0.49 | <0.001 |
| F3 | 36 (9.5) | 10.0 (9.8–10) | 9.2 (8.4–10.3) | 0.30 | 0.081 |
| F4 | 22 (5.8) | 11.9 (11.4–16.3) | 12.5 (10.4–16.1) | 0.77 | <0.001 |
| BMI groups | |||||
| Normal weight | 75 (19.7) | 5.3 (4.5–6.8) | 5.8 (4.8–7.9) | 0.65 | <0.001 |
| Overweight | 78 (20.6) | 4.4 (3.7–5.3) | 4.7 (4.0–5.3) | 0.70 | <0.001 |
| Obese | 227 (59.7) | 4.6 (3.9–5.4) | 5.0 (3.9–6.1) | 0.89 | <0.001 |
TE; Transient Elastography, LSM; Liver Stiffness Measurement, IQR; Interquartile Range, BMI; Body Mass Index.
Table 2 presents the UAP measurements by FibroTouch® across different steatosis stages. The mean UAP by FibroTouch® was 278 ± 44 dB/m, correlating significantly with FibroScan® CAP values across all steatosis stages (rho=0.74, p < 0.001), with varying degrees of correlation strength (S0: rho=0.50, p < 0.001; S1: rho=0.22, p = 0.050; S2: rho=0.13, p = 0.211; S3: rho=0.42, p < 0.001) (Fig. 3). Bland-Altman analysis indicated mean differences and agreement limits across stages, showing a mean difference for all patients of 7.6 ± 34.9 dB/m (Supplementary Fig. 1). Correlations remained significant across BMI categories: normal weight (rho=0.74, p < 0.001), overweight (rho=0.69, p < 0.001), and obese (rho=0.63, p < 0.001) (Table 2).
3.4Liver stiffness measurement by fibroscan® and fibrotouch®Table 3 shows LSM measurements by FibroScan® and FibroTouch® across different fibrosis stages. The median LSM by FibroTouch® was 5.6 kPa (IQR 4.6–7.3), showing strong correlation with FibroScan® LSM (rho=0.87, p < 0.001) (Fig. 4). Significant correlations were observed for fibrosis stages F0-F1 (rho=0.52), F2 (rho=0.49), and F4 (rho=0.77), except for F3 (rho=0.30, p = 0.081). Bland-Altman analysis showed mean differences were significant across stages, except for F4, indicating close agreement between the two methods (Supplementary Fig. 2). Strong correlations between FibroScan® and FibroTouch® LSM were found across BMI groups: normal weight (rho=0.65, p < 0.001), overweight (rho=0.70, p < 0.001), and obese (rho=0.89, p < 0.001) (Table 4).
Correlation of clinical parameters and non-invasive fibrosis markers with FibroTouch® UAP and LSM, and FibroScan® CAP and LSM.
| (n = 380) | FibroTouch® UAP | FibroScan® CAP | LSM by FibroTouch® | LSM by FibroScan® | |||||
|---|---|---|---|---|---|---|---|---|---|
| Correlation value (rho) | p-value | Correlation value (rho) | p-value | Correlation value (rho) | p-value | Correlation value (rho) | p-value | ||
| Clinical parameters | |||||||||
| Age | −0.20 | <0.001 | −0.14 | 0.005 | −0.04 | 0.501 | 0.04 | 0.501 | |
| Platelets count (x 109/L) | 0.14 | 0.018 | 0.11 | 0.058 | 0.04 | 0.447 | 0.001 | 0.984 | |
| Aspartate Transferase (AST) (IU/L) | 0.19 | <0.001 | 0.15 | 0.010 | 0.35 | <0.001 | 0.35 | <0.001 | |
| Alanine Transaminase (ALT) (IU/L) | 0.33 | <0.001 | 0.27 | <0.001 | 0.32 | <0.001 | 0.33 | <0.001 | |
| Gamma-glutamyl Transferase (GGT) (IU/L) | 0.06 | 0.453 | 0.08 | 0.320 | 0.07 | 0.398 | 0.14 | 0.078 | |
| Alkaline Phosphatase (ALP) (IU/L) | −0.02 | 0.797 | −0.001 | 0.982 | −0.04 | 0.549 | −0.03 | 0.650 | |
| Albumin (g/dL) | 0.23 | <0.001 | 0.22 | <0.001 | −0.11 | 0.091 | −0.09 | 0.146 | |
| Fibrosis scores | |||||||||
| Fibrosis-4 Index (FIB-4) | −0.25 | <0.001 | −0.24 | <0.001 | 0.08 | 0.183 | 0.12 | 0.035 | |
| AST to Platelet Ratio Index (APRI) | −0.13 | 0.031 | −0.10 | 0.082 | −0.02 | 0.691 | 0.01 | 0.808 | |
| AST/ALT ratio | −0.40 | <0.001 | −0.40 | <0.001 | −0.16 | 0.004 | −0.21 | <0.001 | |
| SAFE (Steatosis-associated Fibrosis Estimator) Score | 0.10 | 0.217 | 0.09 | 0.241 | 0.31 | <0.001 | 0.40 | <0.001 | |
| HSI (Hepatic Steatosis Index) | 0.54 | <0.001 | 0.54 | <0.001 | 0.38 | <0.001 | 0.41 | <0.001 | |
CAP; Controlled Attenuation Parameter, SD; Standard Deviation, UAP; Ultrasound Attenuation Parameter, LSM; Liver Stiffness Measurement, IQR; Interquartile Range.
Hepatic steatosis measured by FibroTouch® UAP showed a weak negative correlation with age and weak positive correlations with platelets count, AST, and albumin. It also showed a moderate positive correlation with ALT. For non-invasive fibrosis markers, FibroTouch® UAP showed weak negative correlations with FIB-4 and APRI, a moderate negative correlation with the AST/ALT ratio, and a strong positive correlation with HSI. There was no correlation between FibroTouch® UAP and GGT, ALP, or SAFE score (Table 4).
3.6Correlation of LSM by fibrotouch® with clinical parameters and non-invasive fibrosis markersLSM measured by FibroTouch® showed a moderate positive correlation with AST and ALT, but no significant correlation with platelet count, GGT, ALP, or albumin. For non-invasive fibrosis markers, LSM measured by FibroTouch® showed a weak negative correlation with the AST/ALT ratio and a moderate positive correlation with the SAFE score and HSI. However, LSM measured by FibroTouch® did not significantly correlate with FIB-4 or APRI (Table 4).
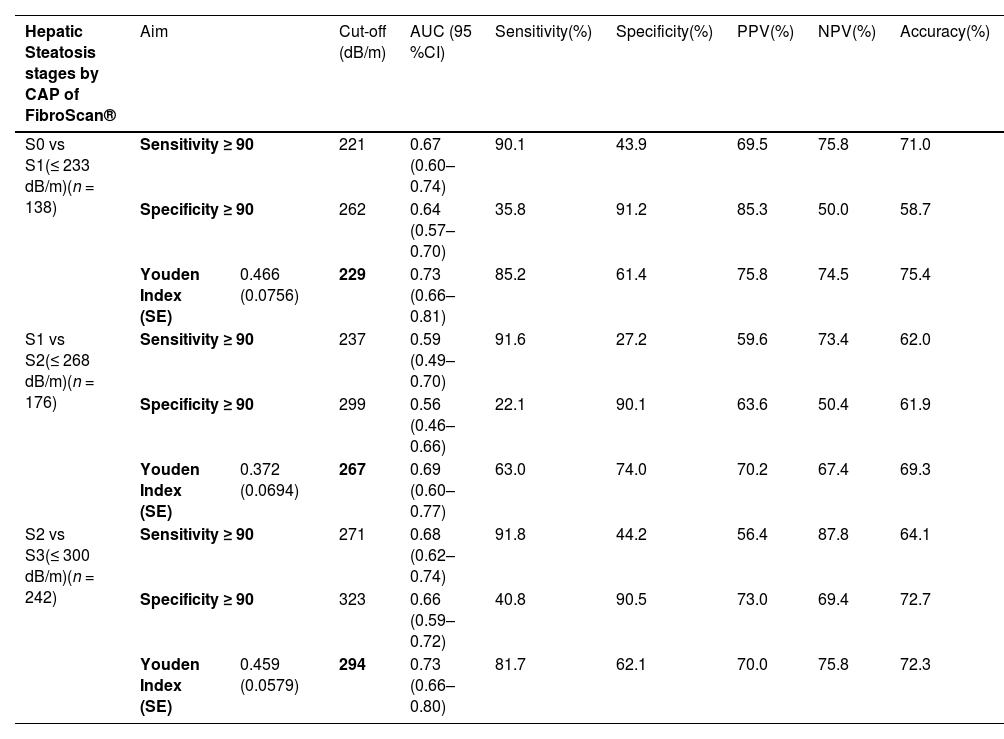
3.7Optimal cut-off values of fibrotouch® UAPIn our study, we evaluated UAP cutoffs for each steatosis stage using three aims: sensitivity ≥90 %, specificity ≥90 %, and Youden Index (Table 5). The optimal FibroTouch® UAP cutoffs identified were 229 dB/m for differentiating S0 from S1, providing 85.2 % sensitivity and 61.4 % specificity with a Youden Index of 0.466; 267 dB/m for S1 vs. S2, achieving 63.0 % sensitivity and 74.0 % specificity with a Youden Index of 0.372; and 294 dB/m for S2 vs. S3, yielding 81.7 % sensitivity and 62.1 % specificity with a Youden Index of 0.459.
Proposed optimal cutoffs of UAP and LSM by FibroTouch® with CAP and LSM of FibroScan® as a reference standard.
| Hepatic Steatosis stages by CAP of FibroScan® | Aim | Cut-off (dB/m) | AUC (95 %CI) | Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | Accuracy(%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| S0 vs S1(≤ 233 dB/m)(n = 138) | Sensitivity ≥ 90 | 221 | 0.67 (0.60–0.74) | 90.1 | 43.9 | 69.5 | 75.8 | 71.0 | ||
| Specificity ≥ 90 | 262 | 0.64 (0.57–0.70) | 35.8 | 91.2 | 85.3 | 50.0 | 58.7 | |||
| Youden Index (SE) | 0.466 (0.0756) | 229 | 0.73 (0.66–0.81) | 85.2 | 61.4 | 75.8 | 74.5 | 75.4 | ||
| S1 vs S2(≤ 268 dB/m)(n = 176) | Sensitivity ≥ 90 | 237 | 0.59 (0.49–0.70) | 91.6 | 27.2 | 59.6 | 73.4 | 62.0 | ||
| Specificity ≥ 90 | 299 | 0.56 (0.46–0.66) | 22.1 | 90.1 | 63.6 | 50.4 | 61.9 | |||
| Youden Index (SE) | 0.372 (0.0694) | 267 | 0.69 (0.60–0.77) | 63.0 | 74.0 | 70.2 | 67.4 | 69.3 | ||
| S2 vs S3(≤ 300 dB/m)(n = 242) | Sensitivity ≥ 90 | 271 | 0.68 (0.62–0.74) | 91.8 | 44.2 | 56.4 | 87.8 | 64.1 | ||
| Specificity ≥ 90 | 323 | 0.66 (0.59–0.72) | 40.8 | 90.5 | 73.0 | 69.4 | 72.7 | |||
| Youden Index (SE) | 0.459 (0.0579) | 294 | 0.73 (0.66–0.80) | 81.7 | 62.1 | 70.0 | 75.8 | 72.3 | ||
| Fibrosis stages by VCTE of FibroScan® | Aim | Cut-off (kPa) | AUC (95 %CI) | Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | Accuracy(%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| F0–F1 vs F2(≤ 5.7 kPa)(n = 323) | Sensitivity ≥ 90 | 5.3 | 0.77 (0.71–0.84) | 91.5 | 62.7 | 52.4 | 94.1 | 72.5 | ||
| Specificity ≥ 90 | 6.8 | 0.71 (0.64–0.78) | 51.2 | 90.5 | 64.8 | 84.3 | 82.1 | |||
| Youden Index (SE) | 0.581 (0.0513) | 6.0 | 0.79 (0.72–0.86) | 80.0 | 78.0 | 52.2 | 93.1 | 78.3 | ||
| F2 vs F3(≤ 7.8 kPa)(n = 118) | Sensitivity ≥ 90 | 7.5 | 0.77 (0.68–0.86) | 91.7 | 63.4 | 57.4 | 93.1 | 74.6 | ||
| Specificity ≥ 90 | 9.6 | 0.67 (0.58–0.76) | 44.4 | 90.2 | 64.3 | 78.1 | 77.1 | |||
| Youden Index (SE) | 0.621 (0.0717) | 7.9 | 0.81 (0.73–0.89) | 89.0 | 73.0 | 56.2 | 94.4 | 77.1 | ||
| F3 vs F4(≤ 10.2 kPa)(n = 57) | Sensitivity ≥ 90 | 9.2 | 0.70 (0.60–0.80) | 90.5 | 50.0 | 46.2 | 92.3 | 63.2 | ||
| Specificity ≥ 90 | 11.6 | 0.74 (0.63–0.85) | 57.1 | 91.7 | 66.7 | 87.5 | 80.7 | |||
| Youden Index (SE) | 0.548 (0.1165) | 10.6 | 0.77 (0.66–0.89) | 71.0 | 83.0 | 68.4 | 84.6 | 80.7 | ||
CAP; Controlled Attenuation Parameter, UAP; Ultrasound Attenuation Parameter, LSM; Liver Stiffness Measurement, CI; Confidence Interval, PPV; Positive Predictive Value, NPV; Negative Predictive Value, SE; Standard Error, VCTE; Vibration-controlled Transient Elastography.
Supplementary Table 1 compared FibroTouch® UAP cutoffs from previous studies [22,29,30] and Hisky Medical's recommended cutoffs in our cohort. Qu et al.’s cutoffs showed moderate sensitivity and specificity, with the best performance for S2 vs. S3 at 82.1 % sensitivity and 61.3 % specificity, with a cutoff of 294 dB/m. Yu et al.’s cutoffs had high sensitivity for S0 vs. S1 but lower specificity across stages, particularly with 45.8 % specificity for S0 vs. S1. Zhu et al.’s cutoffs displayed high sensitivity but lower specificity, especially with 100 % sensitivity and 44.2 % specificity for S0 vs S1. The company Hisky Medical's cutoffs achieved balanced performance, with a sensitivity of 70.4 % and specificity of 70.2 % for S0 vs S1, and relatively better accuracy in our cohort compared to other studies.
3.8Optimal cut-off values of LSM by fibrotouch®Table 5 detailed AUCs and the diagnostic performance of LSM cutoff values optimized using Youden's index, with a sensitivity of 90 % or a specificity of 90 %, for diagnosing fibrosis stages (F0–F1 vs. F2, F2 vs. F3, and F3 vs. F4). For F0-F1 vs. F2, the optimal cutoff was 6.0 kPa, yielding 80 % sensitivity and 78 % specificity. For F2 vs. F3, the cutoff was 7.9 kPa, providing 80 % sensitivity and 73 % specificity. For F3 vs. F4, the best cutoff was 10.6 kPa, achieving 71 % sensitivity and 83 % specificity.
The performance of FibroTouch® LSM cutoffs for hepatic fibrosis stages was evaluated using criteria from previous studies [29,30] and company recommendations. The 6.0 kPa cutoff for F0-F1 vs. F2 demonstrated comparable sensitivity to that of Qu et al. (81.8 %) and Hisky Medical (80.8 %), though with slightly lower specificity. The 7.9 kPa cutoff for F2 vs. F3 in our study provided balanced sensitivity and specificity, showing superior accuracy. For F3 vs. F4, the 10.6 kPa cutoff achieved moderate sensitivity (71.0 %) and specificity (83.0 %), similar to Qu et al. but with improved overall accuracy (Supplementary Table 1).
3.9Concordance between fibrotouch® and fibroscan®The confusion matrices in Tables 6 and 7 detail the concordance between FibroTouch® and FibroScan® for assessing hepatic steatosis and fibrosis stages. A discordance rate of 33.7 % between FibroTouch® UAP and FibroScan® CAP was observed, indicating some discrepancies in steatosis staging (Table 6a). When stratified by BMI, the discordance percentages were 24.0 % for normal weight, 33.3 % for overweight, and 37.0 % for obese groups (Table 6b–d). The Chi-square test for homogeneity showed no significant difference in discordance proportions across BMI categories (χ²=4.27, p = 0.118), suggesting that BMI did not significantly impact the discordance rates between FibroScan® CAP and FibroTouch® UAP. The κ statistic demonstrated fair to moderate agreement between FibroScan® CAP and FibroTouch® UAP for steatosis stages across different BMI groups: normal weight (κ=0.42), overweight group (κ=0.46), and obese group (κ=0.40). The weighted Kappa for steatosis stages was 0.58, indicating moderate overall agreement (Table 6).
Confusion matrix of Fibroscan® CAP and FibroTouch® UAP for the entire cohort (a), normal BMI group (b), overweight group (c) and obese group (d).
| (a) entire cohort | Steatosis stages by FibroTouch® UAP | Total | |||
|---|---|---|---|---|---|
| S0-S1 (n) | S2 (n) | S3 (n) | |||
| Steatosis stages by FibroScan® CAP | S0-S1 (n) | 115 | 13 | 10 | 138 |
| S2 (n) | 38 | 34 | 23 | 95 | |
| S3 (n) | 12 | 32 | 103 | 147 | |
| Total | 165 | 79 | 136 | 380 | |
| Discordance Percentage | 128 (33.7 %) | ||||
| Weighted Kappa (κ) | 0.58 | ||||
| (b) normal weight | Steatosis stages by FibroTouch® UAP | Total | |||
|---|---|---|---|---|---|
| S0-S1 (n) | S2 (n) | S3 (n) | |||
| Steatosis stages by FibroScan® CAP | S0-S1 (n) | 50 | 1 | 1 | 52 |
| S2 (n) | 9 | 4 | 1 | 14 | |
| S3 (n) | 1 | 5 | 3 | 9 | |
| Total | 60 | 10 | 5 | 75 | |
| Discordance Percentage | 18 (24.0 %) | ||||
| Kappa (κ) | 0.42 | ||||
| (c) overweight | Steatosis stages by FibroTouch® UAP | Total | |||
|---|---|---|---|---|---|
| S0-S1 (n) | S2 (n) | S3 (n) | |||
| Steatosis stages by FibroScan® CAP | S0-S1 (n) | 32 | 5 | 1 | 38 |
| S2 (n) | 8 | 12 | 2 | 22 | |
| S3 (n) | 1 | 9 | 8 | 18 | |
| Total | 41 | 26 | 11 | 78 | |
| Discordance Percentage | 26 (33.3 %) | ||||
| Kappa (κ) | 0.46 | ||||
| (d) obese | Steatosis stages by FibroTouch® UAP | Total | |||
|---|---|---|---|---|---|
| S0-S1 (n) | S2 (n) | S3 (n) | |||
| Steatosis stages by FibroScan® CAP | S0-S1 (n) | 33 | 7 | 8 | 48 |
| S2 (n) | 21 | 18 | 20 | 59 | |
| S3 (n) | 10 | 18 | 92 | 120 | |
| Total | 64 | 43 | 120 | 227 | |
| Discordance Percentage | 84 (37.0 %) | ||||
| Kappa (κ) | 0.40 | ||||
CAP; Controlled Attenuation Parameter, UAP; Ultrasound Attenuation Parameter, BMI; Body Mass Index.
Confusion matrix of LSM by Fibroscan® and FibroTouch for the entire cohort (a), normal BMI group (b), overweight group (c) and obese group (d).
| (a) entire cohort | Fibrosis stages by FibroTouch® | Total | |||
|---|---|---|---|---|---|
| F0-F1 (n) | F2 (n) | F3-F4 (n) | |||
| Steatosis stages by FibroScan® CAP | F0-F1 (n) | 195 | 43 | 3 | 241 |
| F2 (n) | 20 | 40 | 22 | 82 | |
| F3-F4 (n) | 3 | 3 | 51 | 57 | |
| Total | 218 | 86 | 76 | 380 | |
| Discordance Percentage | 94 (24.7 %) | ||||
| Weighted Kappa (κ) | 0.66 | ||||
| (b) normal weight | Fibrosis stages by FibroTouch® | Total | |||
|---|---|---|---|---|---|
| F0-F1 (n) | F2 (n) | F3-F4 (n) | |||
| Fibrosis stages by Fibroscan® | F0-F1 (n) | 58 | 7 | 0 | 65 |
| F2 (n) | 5 | 3 | 0 | 8 | |
| F3-F4 (n) | 0 | 0 | 2 | 2 | |
| Total | 63 | 10 | 2 | 75 | |
| Discordance Percentage | 12 (16.0 %) | ||||
| Kappa (κ) | 0.38 | ||||
| (c) overweight | Fibrosis stages by FibroTouch® | Total | |||
|---|---|---|---|---|---|
| F0-F1 (n) | F2 (n) | F3-F4 (n) | |||
| Fibrosis stages by Fibroscan® | F0-F1 (n) | 55 | 11 | 0 | 66 |
| F2 (n) | 3 | 7 | 0 | 10 | |
| F3-F4 (n) | 0 | 0 | 2 | 2 | |
| Total | 58 | 18 | 2 | 78 | |
| Discordance Percentage | 14 (17.9 %) | ||||
| Kappa (κ) | 0.47 | ||||
| (d) obese | Fibrosis stages by FibroTouch® | Total | |||
|---|---|---|---|---|---|
| F0-F1 (n) | F2 (n) | F3-F4 (n) | |||
| Fibrosis stages by Fibroscan® | F0-F1 (n) | 82 | 25 | 3 | 110 |
| F2 (n) | 12 | 30 | 22 | 64 | |
| F3-F4 (n) | 3 | 3 | 47 | 53 | |
| Total | 97 | 58 | 72 | 227 | |
| Discordance Percentage | 68 (30.0 %) | ||||
| Kappa (κ) | 0.54 | ||||
CAP; Controlled Attenuation Parameter, UAP; Ultrasound Attenuation Parameter, BMI; Body Mass Index.
For LSM, the whole cohort had a discordance rate of 24.7 % between FibroTouch® and FibroScan® for fibrosis staging (Table 7a). The discordance percentages varied significantly across the BMI group, i.e., 16.0 %, 17.9 %, and 30.0 % for normal weight, overweight and obese groups, respectively, Table 7b–d, χ²=8.33, p = 0.016. This indicated that BMI significantly impacted the discordance rates of staging fibrosis by the two machines. The κ statistic also showed fair to moderate agreement between FibroScan® and FibroTouch® LSM, with κ values of 0.38 for normal weight, 0.47 for overweight, and 0.54 for obese group. The weighted Kappa for fibrosis stages was 0.66, indicating substantial overall agreement (Table 7).
4DiscussionNon-invasive assessment of hepatic steatosis and fibrosis is vital in MASLD patients for treatment decisions, disease prognosis, and monitoring of disease progression and treatment response [4,9]. This cross-sectional cohort study compared FibroTouch® and FibroScan® for assessing hepatic steatosis and fibrosis in MASLD patients and showed strong correlations between their measurements.
FibroTouch® UAP values correlated positively with FibroScan® CAP values across most steatosis stages, except S2, aligning with previous findings on NAFLD patients [29]. Optimal UAP cutoffs were 229 dB/m for S0 vs. S1, 267 dB/m for S1 vs. S2, and 294 dB/m for S2 vs. S3, outperforming cutoffs previous studies by Qu et al., Yu et al., and Zhu et al., and similar to Hisky Medical's recommendations for S1 vs. S2 and S2 vs. S3. The 267 dB/m cutoff for S1 vs. S2 showed lower AUC and accuracy, indicating challenges in distinguishing these stages. A study on MASLD patients in China also found similar challenges with varied UAP cutoffs for steatosis staging using biopsy as the reference [30]. To better understand FibroTouch® in hepatic steatosis assessment, we included a comparison table summarizing UAP cutoffs from our study and others [21,29,30], highlighting variations of cutoffs in diagnosing steatosis stages in Asian populations (Supplementary Table 1). We propose UAP cutoffs of 229 dB/m to exclude healthy individuals (S0) and 294 dB/m to identify severe steatosis (S3) in the Thai population, though these require validation against liver histology in diverse populations, especially in Western and Hispanic populations.
FibroTouch® UAP showed significant correlations with platelets, AST, ALT, and albumin, aligning with a previous Chinese study [14], and it was also strongly correlated with HIS (rho=0.54, p < 0.001), which is an efficient MASLD screening tool with a sensitivity of 93.1 % for ruling out and a specificity of 92.4 % for detecting MASLD [31]. A study in Spain highlighted the higher risk of liver disease progression with greater hepatic steatosis, emphasizing the clinical importance of identifying severe steatosis over distinguishing S1 from S2 [32,33]. While ultrasound is commonly used for screening, its sensitivity of 49.1 % and specificity of 75 % are limited, especially in obese individuals [34,35], making FibroTouch® a superior option. However, FibroTouch®’s suboptimal performance in distinguishing S1 and S2 likely stems from overlapping steatosis characteristics and variability in patient factors like BMI and liver heterogeneity [14,30]. Despite BMI not significantly affecting discordance rates, a high discordance rate (33.7 %) between FibroTouch® UAP and FibroScan® CAP indicates discrepancies in steatosis staging, with a moderate agreement with weighted κ of 0.58 and the lowest κ (0.40) observed in the obese group. FibroScan®’s use of specialized probes for different BMIs might enhance sensitivity and specificity, reducing measurement overlap. While image-guided positioning in FibroTouch® may reduce errors and improve accuracy, FibroScan®’s tailored approach for varying BMIs likely contributes to its superior ability to distinguish steatosis stages [14,30]. Despite these challenges, FibroTouch® effectively excluded S0 and identified S3, surpassing ultrasound, although discrepancies between FibroTouch® UAP and FibroScan® CAP highlight the need for further refinement.
Our study identified optimal FibroTouch® LSM cutoffs of 6.0 kPa for F0-F1 vs. F2, 7.9 kPa for F2 vs. F3, and 10.6 kPa for F3 vs. F4, outperforming Qu et al. and Yu et al. in sensitivity, specificity, and AUC. Although our cutoffs differed from Hisky Medical's recommendations, they showed comparable diagnostic performance (Supplementary Table 1). In our study, the 7.9 kPa cutoff for F2 vs. F3 outperformed the 10.6 kPa cutoff for F3 vs. F4. The discordance rate between FibroScan® and FibroTouch® was highest among obese patients (30.0 %), with BMI significantly impacting discordance rates. Despite this, κ 0.66 indicated reasonable moderate agreement between the two machines. A previous study highlighted increased mortality in F3 and F4, classified as compensated advanced chronic liver disease (cACLD) patients, though distinguishing between these stages may have limited clinical impact [36]. A Japanese study also noted a poorer prognosis in NASH patients with advanced fibrosis (F3-F4) [37]. FibroTouch® could be useful for identifying cACLD patients, as evidenced by our comparison with LSM cutoffs of other studies (Supplementary Table 1). Additionally, FibroTouch® LSM moderately correlated with AST, ALT, and the SAFE score, consistent with previous findings in China on NAFLD, where AUCs exceeded 0.8 for fibrosis stages [14]. Moreover, LSM by FibroTouch® was also moderately associated with the SAFE score, a new non-invasive fibrosis marker that uses widely available variables to estimate liver fibrosis, effectively distinguished F0–F1 from ≥F2, surpassing FIB-4 and NAFLD Fibrosis Scores [38]. Chen et al. found significant correlations between FibroScan®, FibroTouch®, and fibrosis scores, with FibroTouch® AUCs exceeding 0.8 for mild and severe fibrosis [39]. Zeng et al.’s study with 1621 patients validated the strong correlation between FibroTouch® and FibroScan® LSM values (rho=0.645) [20]. Furthermore, FibroTouch® demonstrated higher inter- and intra-observer reliability than FibroScan® [13], reinforcing its utility as a reliable and effective tool for assessing liver fibrosis in MASLD patients.
Our study has some limitations that warrant acknowledgment. Firstly, we did not use liver biopsy as the reference standard. Instead, we justified using FibroScan® based on its demonstrated accuracy and reproducibility in ruling out advanced liver fibrosis in both adult and pediatric NAFLD patients [26,40]. A recent study introduced the CAP-BMI-AST (CBST) score, which combined UAP from FibroTouch® with clinical parameters to improve the diagnostic performance of CAP for assessing liver fat content [41]. This new CBST score, validated against MRI-PDFF measurements and verified in two cohorts, serves as an accurate tool for diagnosing different degrees of liver fat content among MASLD patients. Therefore, using FibroScan® as a reference is justifiable for assessing the diagnostic performance of FibroTouch® without needing liver biopsy results. Secondly, our study included only a small proportion of patients with F3-F4 fibrosis, comprising approximately 15 % of the entire cohort, which may influence the interpretation of the results. Nevertheless, the relatively high and significant correlation between LSM measurements obtained from FibroScan® and FibroTouch® among F4 patients provided valuable insight. Lastly, our study exclusively focused on steatotic liver disease (SLD) patients, excluding those with chronic hepatitis B and C. While previous studies evaluated FibroTouch® primarily in HBV cohorts [42,43], our unique methodology contributes valuable evidence supporting the clinical application of FibroTouch® for MASLD, an emerging chronic liver disease. Although this approach highlights our study's uniqueness, according to the new definition of MASLD, excluding chronic hepatitis B and C is not necessary.
Previous studies show that FibroTouch® and FibroScan® have comparable accuracy for staging fibrosis in chronic liver disease, with differences due to their operating mechanisms [12,15–17]. FibroScan® utilizes VCTE with different probes tailored to various body types, improving accuracy in obese patients by compensating for the attenuation caused by subcutaneous adipose tissue. Its single-element ultrasound transducer produces consistent measurements of liver stiffness and steatosis with well-established protocols and validation across diverse populations. Consequently, using FibroScan® may require different probes for patients with varying BMIs [44,45] (Fig. 2a). Conversely, FibroTouch® employs a 2.5 MHz probe with a focus on image-guided positioning and a larger sampling volume. While this design minimizes interference from anatomical structures and enhances the speed and success rate of measurements, it uses a single probe frequency, which may limit its accuracy in patients with higher BMIs [14,19] (Fig. 2a). In our study, the correlations between UAP and CAP values, as well as LSM values from both devices, remained significant across different BMI groups. Moreover, the differing ranges of UAP (90–450 dB/m) and CAP (100–400 dB/m) contribute to discrepancies in steatosis staging [46]. Therefore, FibroTouch® may offer distinct advantages in quantifying liver fat content across different BMI groups using a single probe. This limitation, combined with FibroScan®’s multi-probe approach and established reference standards, makes FibroScan® more reliable for differentiating steatosis stages, particularly in obese individuals. However, the primary limitation of FibroScan® is its relatively high instrument cost (US $50,000) and associated annual maintenance fees (US $8500), along with the substantial expense for patients [19]. Justifying the application of a screening policy by health authorities often relies on the ten criteria outlined by Wilson and Jungner, where cost-effectiveness is deemed crucial [47]. Therefore, given the expensive nature of using FibroScan® as a screening tool, further research is strongly recommended to conduct cost-effectiveness analyses between FibroScan® and FibroTouch®. Consequently, while FibroScan® remains the preferred reference standard for evaluating liver steatosis and fibrosis, FibroTouch® may serve as a viable alternative.
5ConclusionsFibroTouch® may be valuable for diagnosing MASLD, showing comparable accuracy to FibroScan®. Study cutoffs may guide diagnosis but need validation against liver histology, and future research should explore molecular mechanisms to enhance diagnostic performance.
Funding supportThis work was supported by the Second Century Fund (C2F) of Chulalongkorn University, Bangkok, Thailand. No funding was received from any pharmaceutical company. Dr. Soe Thiha Maung gratefully acknowledges the support from the Second Century Fund (C2F) of Chulalongkorn University for his Doctor of Philosophy (Ph.D.) in Clinical Sciences (International Program) and for his Clinical Fellowship at the Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Thanikan Sukaram also acknowledges the support from the Second Century Fund (C2F) of Chulalongkorn University for her Doctor of Philosophy (Ph.D.) in Medical Sciences.