Idiosyncratic drug-induced liver injury (DILI) caused by xenobiotics (drugs, herbals and dietary supplements) is an uncommon cause of liver disease presenting with a wide range of phenotypes and disease severity, acute hepatitis mimicking viral hepatitis to autoimmune hepatitis, steatosis, fibrosis or rare chronic vascular syndromes. Disease severity ranges from asymptomatic liver test abnormalities to acute liver failure. DILI has been traditionally classified in predictable or intrinsic (dose-related) or unpredictable (not dose-related) mechanisms. Few prospective studies are assessing the real prevalence and incidence of hepatotoxicity in the general population. DILI registries represent useful networks used for the study of liver toxicity, aimed at improving the understanding of causes, phenotypes, natural history, and standardized definitions of hepatotoxicity. Although most of the registries do not carry out population-based studies, they may provide important data related to the prevalence of DILI, and also may be useful to compare features from different countries. With the support of the Spanish Registry of Hepatotoxicity, our Latin American Registry (LATINDILI) was created in 2011, and more than 350 DILI patients have been recruited to date. This position paper describes the more frequent drugs and herbs-induced DILI in Latin America, mainly focusing on several features of responsible medicaments. Also, we highlighted the most critical points on the management of hepatotoxicity in general and those based on findings from our Latin American experience in particular.
drug-induced liver injury
genome-wide association studies
alanineaminotransferase
aspartateaminotransferase
alkaline phosphatase
gamma-glutamyl transferase
total bilirubin
international classification of diseases
drug-induced liver injury network
herbal-induced liver injury
herbal dietary supplement
androgenic anabolic steroids
Latinamerican registry of hepatotoxicity
acute liver failure
non-alcoholic fatty liver disease
methotrexate
Roussel Uclaf Assessment Method
acetaminopnen
high mobility group box-1
macrophage colony-stimulating factor receptor 1
glycodeoxycholic acid
virus
hepatitis B virus
hepatitis delta virus
hepatitis C virus
hepatitis E virus
cytomegalovirus
Epstein-Barr virus
autoimmune hepatitis
Panamerican Health Organization
N-acetylcysteine
checkpoint inhibitors
ursodeoxycholic acid
Drug-induced liver injury (DILI) represents a challenging cause of liver disease since around 1100 drugs have been involved in liver damage and hepatotoxicity and its clinical course can mimic all forms of acute and chronic liver disease. Although most DILI episodes are self-limited with complete resolution after the withdrawal of the culprit agent, hepatotoxicity is the most common cause of acute liver failure in several countries [1]
Interestingly, due to its broad spectrum of clinical and histological presentation, DILI may be mimicked by many other liver diseases. Hence, in the pharmacovigilance scenario where early suspicion is important, and overdiagnosis can occur; a thorough evaluation typically shows that 50% of cases initially ascribed as hepatotoxic events by the spontaneous reporting system are caused by non-DILI liver disease [2].
DILI has been traditionally classified in predictable or intrinsic (dose-related) or unpredictable (not dose-related) mechanisms. Unpredictable reactions are also described as idiosyncratic, either immune-mediated hypersensitivity or nonimmune reactions [3]. Intrinsic DILI is typically dose-related and occurs in a large proportion of individuals exposed to the drug (predictable), and onset is within a short time (hours to days). Idiosyncratic DILI is usually not dose-related, although a dose threshold of 50–100mg/day is usually required, occurs in only a small proportion of exposed individuals (unpredictable) and exhibits a variable latency to onset of days to weeks.
The idiosyncratic DILI is influenced by three factors: a drug that can generate toxic radicals in the liver, a genetically susceptible subject and the intervention of other host and environmental factors. These two last risk factors can impact DILI according to both different ethnic and geographical areas.
DILI pathogenesis is complex, depending on the interaction of drug physicochemical properties and host factors. DILI initiation is assumed to be hepatocyte exposure to some form of stress, most likely involving reactive metabolites, mitochondrial dysfunction and oxidative stress [3]. Reactive metabolites are formed during drug metabolism, usually through cytochrome P450 (CYP450) mediated reactions (phase I). Drugs being CYP450 substrates have a significantly higher risk of causing DILI [4]. On the other hand, genome-wide association studies (GWAS) have identified several alleles from the major histocompatibility complex system, indicating an essential role of the DILI pathogenesis's adaptive immune system. Genetic variations in the HLA region on chromosome 6 have been identified with genome-wide significant differences between DILI cases and controls [5,6]. However, most identified HLA risk alleles have low predictive value and are subsequently limited clinical use for genetic screening before prescription to prevent liver injury.
Thanks to creating prospective registries of hepatotoxicity, DILI features can be better understood according to different countries.
The rationale for writing a position paper instead of specific practical clinical guidelines is due to several reasons follows (a) the lack of multicenter population-based epidemiological studies in LA, (b) the absence of systematic revisions and meta-analysis, (c) the lack of consistent data on pharmacogenetic studies, (d) low level of awareness of physician to report DILI cases, (e) distinct regulatory policies according to different countries. This article will focus only on idiosyncratic liver toxicity.
We aim to describe the more frequent drugs and herbs for DILI in Latin America, mainly focusing on several responsible medicaments. We have also developed the most relevant issues on the management of hepatotoxicity in general, and those based on findings from our Latin American experience, in particular, highlighted at the final of each topic.
2Current definition, clinical patterns and severity assessmentLiver injury is usually detected and confirmed by the presence of abnormal biochemical tests mainly including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin (TBL). An international expert working group meeting in 2011 proposed a new definition for DILI that includes (i) ALT elevation ≥5 ULN, (ii) ALP elevation ≥2 ULN (particularly with accompanying elevations in gamma-glutamyltransferase (GGT) concentrations and the absence of known bone pathology driving the increase in ALP level) or (iii) ALT ≥3 ULN and simultaneous elevation of total bilirubin concentration above 2 ULN [7].
The clinical pattern is defined as “hepatocellular” when there is an ALT/ALP ratio higher than or equal to 5 or an ALT greater than five times the LSN. Liver injury is called “cholestatic” when there is an increase of 2 or more times the ALP alone or when ALT/ALP serum activity is two or less. When the ALT/ALP ratio is between 2 and 5, the clinical pattern is called ‘mixed’ [7].
It should be kept in mind that a minor increase in aminotransferases could be due to an adaptive response of the liver when exposed to certain agents. This biochemical situation is usually reversible and should not be classified as DILI [3]. The most paradigmatic example is the initial increase in ALT that occurs with statins, where an increase in liver enzymes is usually a transitory adaptation phenomenon that does not require the suspension of the drug [8]. Similarly, an isolated increase in total bilirubin levels does not qualify as DILI. It can be explained by different alterations in the conjugation of this pigment like happens in sepsis (predominant direct bilirubin) or secondary to Gilbert's Syndrome (an increase of indirect bilirubin) [7]. The same concept should be incorporated regarding an isolated increase of GGT, related to enzymatic reaction. Interestingly, aspartate aminotransferase (AST) yet less specific for the liver may replace ALT, in the absence of known muscle injury.
Regarding DILI severity index, two classifications have been proposed; the US DILIN proposes five grades (mild, moderate, moderate-severe, severe and fatal) taking into account the need for hospitalization [9]. Moreover, the International DILI Expert Working Group's severity index only considers four grades (mild, moderate, severe and fatal/transplantation). The latter classification does not consider hospitalization due to important variability hospitalization indications between different hospitals/medical organizations [7]. This expert meeting report classified severity index as mild: ALT ≥5×ULN or ALP ≥2×ULN, and TBL <2×ULN; Moderate: ALT ≥5×ULN or ALP ≥2×ULN, and TBL ≥2×ULN; Severe: ALT ≥5×ULN or ALP ≥2×ULN, and TBL ≥2×ULN and one of the following: (i) international normalized ratio ≥1.5, (ii) ascites or encephalopathy, disease duration <26 weeks, and absence of underlying cirrhosis, (iii) other organ failure considered to be due to DILI and iv) fatal/transplant: when death or liver transplant occurred.
Point to highlight
- •
Induction of immune tolerance against a drug not necessarily induces a clinical reaction. Consequently, an event referred as “adaptation” is characterized by only mild liver enzymes abnormalities and it does not necessarily have to be followed by the suspension of the incriminated drug. It usually happens with an extensive list of drugs, and the clinician needs to be aware of this issue.
Hepatotoxicity is an uncommon event in clinical practice that makes knowing the true incidence of DILI difficult. One of the most important causes of this statement is that patients at risk of liver toxicity must be followed for a long time. On the other hand, clinical trials are generally underpowered to detect a low incidence of hepatotoxicity. Many of the idiosyncratic adverse events related to the drugs are typically detected many years after the drug has been launched to the market. Although a voluntary reporting system of adverse drug events has been established in many countries, most cases are underreported.
Prospective, retrospective and registry-based studies are the most important methods to obtain epidemiological data on DILI.
3.2Prospective studiesProspective population-based studies are intended to detect DILI by assessing all subjects living in a specific area.
The first population-based study was carried out in a French population, showing an annual incidence rate of DILI of 13.9 cases per 100,000 inhabitants. Twelve per cent of these patients required hospitalization, and 6% of them died [10].
A more recent population and well-designed study from Iceland reported a higher incidence of 19 cases per 100,000 inhabitants per year. DILI was caused by a single prescription medication in 75% of cases, by dietary supplements in 16%, and by multiple agents in 9% of cases [11].
3.3Retrospective studiesDILI retrospective studies aimed to identify liver toxicity on databases have generally used the International Classification of Diseases (ICD-9) codes which have shown low sensitivity and specificity levels. Indeed, causality assessment in these studies is hampered by incomplete data on records [12]. Not surprisingly, retrospective studies show a DILI incidence rate lower than that reported in prospective studies
Robust and confident epidemiological data historically have been lacking in LA. The first evaluation of the profile of DILI in LA comes from the analysis of case reports and series published between 1996 and 2012, describing a total of 176 cases (53 drugs) [13]. Ninety per cent of the cases came from Chile, Argentina, and Colombia, while Peru, Uruguay, Brazil, Mexico, Venezuela, and Cuba accounted for the remaining 10%. The most frequently reported pharmacological groups were non-steroidal anti-inflammatory drugs (NSAIDs) (32%), anti-infective agents (19%), and anti-androgens (16%), mainly cyproterone acetate.
Interestingly, a recent systematic review was undertaken by Santo et al., who analyzed all published case reports on DILI-induced by HDS (herbal dietetic supplements) in LA from 1976 to 2020 [14]. They found 23 cases where centella asiatica, cathmus tinctorious and herbalife were the most common culprit products inducing liver reactions mainly indicated to lose weight. These hepatic events occurred in young women in whom hepatocellular pattern of liver damage was the most common clinical presentation. Besides, ALF linked to a high mortality rate, inadvertent rechallenge and chronic liver disease was also observed.
3.4DILI registriesRegistries are networks used to study hepatotoxicity, aimed at improving the understanding of DILI causes, phenotypes, natural history, and standardized definitions and may better detect early postmarketing liver toxicity. Besides, they may also facilitate the harmonization of clinical studies. Although most of the registries do not carry out population-based studies, they may provide important data related to the prevalence of DILI and may be useful to compare results among different countries.
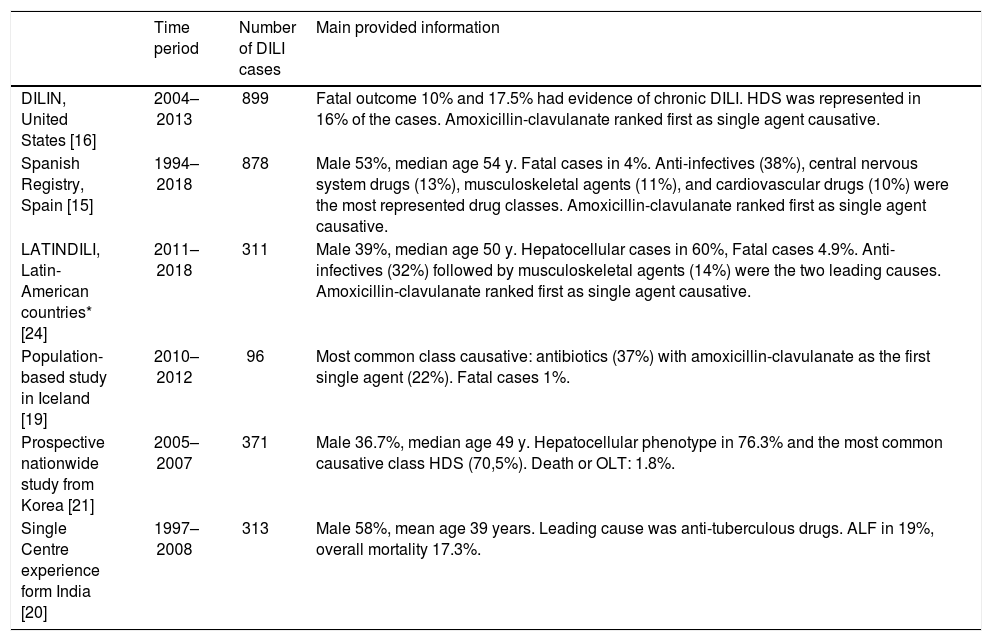
Numerous international DILI registries have been created during the past decade from Spain [15] USA [16], Australia [17], Sweden [18], Iceland [11], India [19], South Korea [20], and Latin America [21] which contributing with the understanding of local hepatotoxicity. Most of them showed anti-infectious agents as the main worldwide cause of DILI (Table 1).
Major DILI registries ongoing worldwide and prospective published studies.
| Time period | Number of DILI cases | Main provided information | |
|---|---|---|---|
| DILIN, United States [16] | 2004–2013 | 899 | Fatal outcome 10% and 17.5% had evidence of chronic DILI. HDS was represented in 16% of the cases. Amoxicillin-clavulanate ranked first as single agent causative. |
| Spanish Registry, Spain [15] | 1994–2018 | 878 | Male 53%, median age 54 y. Fatal cases in 4%. Anti-infectives (38%), central nervous system drugs (13%), musculoskeletal agents (11%), and cardiovascular drugs (10%) were the most represented drug classes. Amoxicillin-clavulanate ranked first as single agent causative. |
| LATINDILI, Latin-American countries* [24] | 2011–2018 | 311 | Male 39%, median age 50 y. Hepatocellular cases in 60%, Fatal cases 4.9%. Anti-infectives (32%) followed by musculoskeletal agents (14%) were the two leading causes. Amoxicillin-clavulanate ranked first as single agent causative. |
| Population-based study in Iceland [19] | 2010–2012 | 96 | Most common class causative: antibiotics (37%) with amoxicillin-clavulanate as the first single agent (22%). Fatal cases 1%. |
| Prospective nationwide study from Korea [21] | 2005–2007 | 371 | Male 36.7%, median age 49 y. Hepatocellular phenotype in 76.3% and the most common causative class HDS (70,5%). Death or OLT: 1.8%. |
| Single Centre experience form India [20] | 1997–2008 | 313 | Male 58%, mean age 39 years. Leading cause was anti-tuberculous drugs. ALF in 19%, overall mortality 17.3%. |
*Argentina, Uruguay, Brazil, Chile, Mexico, Peru, Paraguay, Venezuela, Ecuador and Dominican Republic. ALF, acute liver failure; DILI, drug-induced liver injury; DILIN, Drug-Induced Liver Injury Network; HDS, herbal and dietary supplements; LATINDILIN, Latin-American DILI Network; OLT, Orthotopic Liver Transplantation.
The Spanish DILI Registry was a pioneering project set-up in 1994 that studied DILI patients described its first 460 DILI patients in 2005. In this group, 49% were female, 71% were jaundiced, and 51% were hospitalized. Antimicrobials ranked first as implicated drugs. During the follow-up, 7% died or required liver transplantation [15].
The Drug-Induced Liver Injury Network (DILIN) was created by the National Institutes of Health (NIH) in 2003. Currently, this registry includes eight clinical sites in the United States. When analyzing diagnostic characteristics of 899 patients in this network, 10% per cent of patients died or underwent liver transplantation, and 17% had a chronic liver injury. Pre-existing liver disease was documented in the 89 patients (10%), and mortality was significantly higher in this group as compared with patients with normal liver (16% vs. 5.2%) [16].
The Latin American DILI registry (LATINDILI) was created in 2011 due to a Spanish Registry initiative from Malaga. It merits being a network involving different countries, which have some common roots and substantive disparity regarding ethnicity, prescription patterns, and regulatory policies, among others [21].
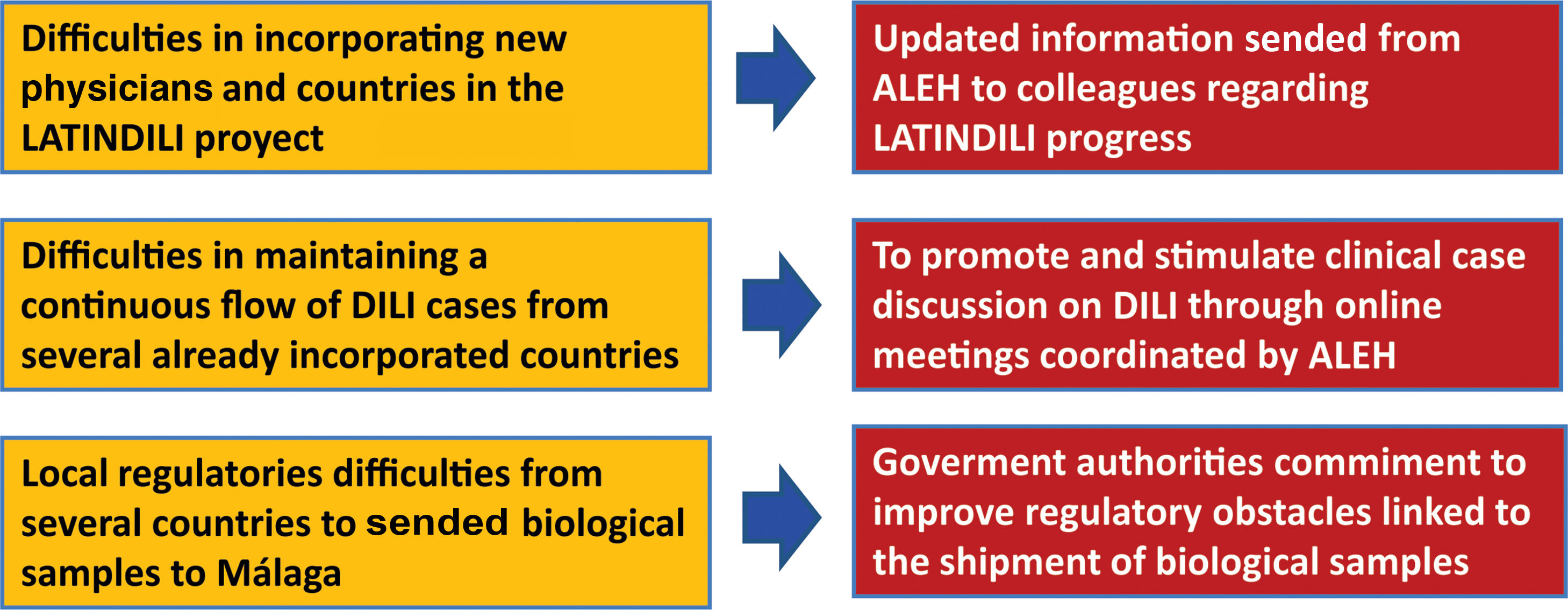
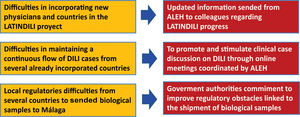
The main goal of the creation of the DILI registry in LA was the prospective and standardized identification of the different manifestations that drug-induced liver disease has in this region, to obtain precious information regarding subject characteristics, most frequently involved drugs or herbal supplements, phenotypic presentations, and outcomes [21–23]. The Asociación Latinoamericana para el Estudio del Hígado – Latin American Association for Study of the Liver (ALEH) has endorsed our LATINDILI project, providing the group excellent visibility in its Website (available online: http://www. https://alehlatam.org) [24]. The physicians contacted and committed to the project are responsible for spreading it in their respective countries and generating—by using the resources most suitable to their local situations—an internal training network for possible DILI cases. Based on suspicion of DILI and following the Spanish model, the treating clinician fills out a standardized form sent to the coordinating physician in each country for a first evaluation, and then to the coordinating centre in Malaga, Spain. The information provided is analyzed, for completeness and the possible association with the drug and drug-drug interactions is evaluated. The causes that were excluded are discussed and, finally, after being evaluated by three independent experts, the event is adjudicated, or not to DILI. Difficulties in recruiting countries to our project and proposed initiative attitudes to solve this problem are shown in Fig. 1.
Several achievements have been attained to date from our network since its creation. One of the most important of them is that this registry has contributed to increasing the knowledge and awareness of DILI disorders in Latin America. We have recruited 390 patients to date where amoxicillin-clavulanic acid is at the top of the list similar to what has been reported by other international registries (unpublished data).
LATINDILI also has recently contributed with the International Drug-Induced Liver Injury consortium in a collaborative international genome-wide association study as a strategy to identify the genetic variations that increase the susceptibility to DILI [25].
Points to highlight
- •
Epidemiology of DILI in LA has never been studied through well-designed studies.
- •
The creation of the DILI network has proven to be useful in recognizing regional characteristics of hepatotoxicity. However, there is not yet a widespread awareness in several Latin American countries about this consortium's existence. This position paper should be a stimulus for clinicians to contribute to our registry sending their DILI cases.
- •
Our Latin American data from LATINDILI continues to grow and helping us to better understand drug behaviour in this region.
- •
To encourage specialists to conduct well-designed epidemiological studies that may answer several remaining questions regarding hepatotoxicity in LA should be our most crucial aim on this topic. Prospective registries are therefore highly recommended.
- •
The possibility of our LATINDILI registry of participating with the international collaborative genome-wide studies opens doors to better pharmacogenetic identification of our Latin American population.
Herbal-induced liver injury (HILI) and herbal and dietary supplement (HDS)-induced liver injury are prominent aspects of drug safety.
There is no discussion on how to define HILI as “herbal-induced liver injury”, but when we define “HDS”, many compounds and non-standardized ingredients are included in this definition, involving anabolic steroids (AAS), which do not belong to the mistakenly called “dietary supplement”. A stricter definition is needed to categorize this topic better. Probably, anabolic steroids should be categorized as “abuse agents” or “hormonal compounds”.
HDS liver toxicity represents a significant public health issue in many regions including in LA due to several reasons: (1) The increasing consumption of HDS and its corresponding HILI risk, (2) popular magazines and websites, which promote herbs as a healthy medication, (3) historical safety reputation, easy access including the Internet, (4) patient frustration with the limitations of traditional medicine, as occurs in common conditions such as obesity or chronic pain. [26].
Sales of herbal and supplements have also increased four-fold in the United States (US) from 1994 to 2014, reflecting the increasing burden of this public health problem. Fifty per cent of the US population has consumed at least one alternative medicine at some point [26].
An analysis by Navarro and coworkers from the DILIN network in the US showed a low frequency of herbal toxicity between 2004–2005, but a duplicated number of cases of both bodybuilding and herbal non-bodybuilding agents was observed between 2007 and 2014 [27]. Similar findings were documented by Medina Cáliz and coworkers, who analyzed a period between 2014 and 2016, where they found an over 20% increase in anabolic agent-induced DILI, headed by stanozolol [28]. These data were obtained from the Spanish registry of hepatotoxicity where they revised 931 cases, of which 856 were cases with a single episode. Of these 856 cases, 32 were due to HDS, and 20 to anabolic steroids. Camellia Sinensis, (traditionally known as green tea), was on the top of the list, followed by herbalife as the two leading causes of herbal toxicity in this network.
HDS and HILI are very concerning issues since regulation of HDS products varies considerably across the world. The FDA does not require manufacturers to register herbal products to have limited information on the number, types, and ingredients of these compounds within the market. Product labels may not provide full disclosure of their ingredients, concentrations, purity, and sources. In contrast with these limitations, Europe has more accurate HILI guidelines based on a European parliament directive, including case by case discussion [29].
It has been documented that HILI is frequently associated with severe liver disease at presentation. Herbal products such as Usnic Acid, OxyELITE-pro, and Hydroxycut, most of them used for losing weight, have received warnings from the FDA for their risk to induce severe liver disease. A dramatic outbreak of liver damage induced by OxiELITE-pro was reported in 8 patients in 2013, where one died, and two of them underwent liver transplantation [30]. Flavocoxid products consisting of plant-derived flavonoids were also recently withdrawn from the market due to severe hepatotoxicity reports [31].
The global prevalence of herbal toxicity is mostly unknown and varies according to different geographical areas. Perhaps, the best estimate for the incidence of HDS-related liver injury comes from the population-based survey carried out in Iceland by Bjornsson and coworkers. [11]. This two-year study revealed that the overall incidence of DILI in 2011 and 2012 was estimated to be 19 cases per 100,000 individuals. In this study, 16% of the cases were attributed to HDS, and almost one-third of patients were jaundiced and hospitalized. These data showed that HDS-related acute liver injury incidence in this study was 3 per 100,000 individuals.
Mercedes Robles and coworkers [32] recently described a different phenotype linked to the larger series of anabolic induced DILI in 25 patients included in the Spanish and LATINDILI registries. This emerging phenotype is characterized by deep jaundice and prolonged cholestasis associated with a renal compromise where the primary implicated agent was stanozolol. The authors found that liver injury mainly developed in young men and its clinical patterns of liver toxicity could be identified as both hepatocellular and cholestatic, at presentation. Most patients were jaundiced, and hospitalization was more frequently needed in the cholestatic group.
Probably these data underrepresents the burden of herbal toxicity in Latin America because these traditional remedies are underreported from countries with a high rate of herbal consumption.
Traditional medicine in Asia is integrated into healthcare systems. There is a market for herbal products as the main source of primary health. Prevalence of HILI is high and varies according to different countries, ranging between 8 and 72% [33].
Interestingly, according to the WHO, herbal contaminants were the most important culprits in HILI in both Western and Eastern countries, including heavy metals, mycotoxins, pesticides, and unlabeled alternative plant species [34]. Unfortunately, a concerning issue that limits the definitive diagnosis of HILI is the shortage of specialized toxicological laboratories capable of analyzing the hidden ingredients culprit of liver toxicity. An interesting prospective study showed that HDS accounted for 16% of DILI cases and this percentage increased during eight years from 7% in 2004–2005 to 19% in 20,102,012 [35]. The fact that contaminants were the main cause of HDS (68% of cases) is worrisome. Green tea, bodybuilding agents and multi-ingredients have been the most frequent culprit agents in this work Bodybuilding supplements are a highly concerning issue because these compounds can be freely purchased over the internet, and they also are mistakenly marketed as a dietary supplement and are therefore available to an underage population.
When clinicians are faced with an elusive culprit of HILI, the only tool for detecting this hidden compound is to carry out chemical and toxicology studies, either in vitro or in vivo, to analyze the chemical components of the ingredients [36].
Points to highlight
- •
Most Latin American countries have high herbs consumption, but the under-reporting cases of HILI is a concerning issue.
- •
This position paper should be a wakeup call to the countries that have not yet joined our registry. To stimulate physicians to send HILI cases to know the real impact of herbs and dietary supplements in LA is one of the most important aims.
- •
Urgent behaviours and stronger health policies should be taken regarding HDS with emphasis on bodybuilding supplements.
The idiosyncratic DILI is thought to be due to the interaction of three factors: a drug that can generate toxic radicals in the liver, a genetically susceptible subject and the intervention of other host and environmental factors [3].
5.1AgeAge has been associated with a higher risk of DILI even though the age cut-off has not been fully defined. A study from Spain showed a higher rate of liver toxicity in patients over 60 years, whereas data from Iceland observed that patients over 70 years showed 41/100,000 episodes of DILI compared with 9/100,000 from people between 9–29 years [11,18].
Our LATINDILI network showed that hepatotoxicity most commonly developed in individuals over 50 years of age (mean age 54 years). We also observed that the highest incidence of DILI was more frequent in individuals who consumed a higher number of medications suggesting that not only age but also other concomitant drugs increased DILI risk (unpublished data).
5.2Ethnicity and genetic factorsDespite an extensive list of well-recognized risk factors for the development of DILI that has been previously described (associated with the drug, the host and the environment), ethnic and underlying liver disease are still poorly studied issues. Although many different ethnic groups are recognized in our region, no studies on DILI susceptibility have been carried out. On the other hand, schistosomiasis as an endemic parasitic disease in several Latin American countries should be discussed as a separate risk factor.
Several categories of ethnic groups have been described within Latin American countries. The majority of these groups are made up of European, African, or Amerindian descent, or a mix of these individuals. It can be divided into categories according to their historical origins: (a) Amerindians: Mayan, Nahua, Huichol, Chibcha, Quechua, Araucanian, Guarani, and Guajito, among others, (b) Criollos or recent European immigrants: of Spanish, French, Portuguese or other descent, (c) Afro-descendants, (d) Mestizos: mulattos, zambos, pardos, among others [37].
Ethnical differences in DILI incidence rates have been noted in the US, but they could partially reflect variations in healthcare insurance and prescription drugs among ethnic populations [16]. Ethnicity as a DILI risk factor could also be an indirect measure of underlying genetic variations. Recent meta-analyses of ethnically different case-control studies on anti-tuberculosis (TB) drug hepatotoxicity have demonstrated that associations to genetic variations in drug-metabolizing genes such as NAT2, CYP2E1, GSTM1 and GSTT1 vary between different ethnic populations [38]. Furthermore, genetic variations in keratin8 and 18 that segregate with ethnic backgrounds predispose to drug-induced ALF [39]. Recently, Chalasani and coworkers [40] showed severity differences in idiosyncratic DILI between African vs. caucasian Americans. These authors stated that the severity of illness tended to be greater in African-Americans than Caucasians as determined by peak mean bilirubin (14.3 vs. 12.8mg/dL), INR (1.9 vs. 1.6) and DILIN severity score (3.0 vs. 2.6). The frequency of severe cutaneous reactions was significantly higher in African-Americans (2.1 vs. 0.36% in Caucasians, p=0.048).
Latin American patients, usually referring to them as “Hispanics”, is a term that is mainly derived from their language rather than their ethnic background and can vary between and within Latin American countries. Hispanic ethnicity also includes ‘mestizo’ (defined in Latin America as individuals with European and Amerindian ancestral background). No studies compared these populations regarding DILI outcome in Latin America. Also, we tend to define the word “indigenous” for the population, which first originated in a particular land. The identification of these people has been a constant and serious problem worldwide. The indigenous people are problematic because there are more than 300 different ethnics within this group, and the information related to the effect of health care among indigenous in Latin American countries is scarce [37].
All these ethnic obstacles are very difficult to overcome for carrying out studies in idiosyncratic DILI. However, one of the most important aims of this position paper is to stimulate Latin American researchers to design epidemiological studies to cover this important topic.
5.3Underlying liver diseaseWhether pre-existing liver disease predisposes to hepatotoxicity or not is still under debate. Viral hepatitis B and C co-infections appear to increase the risk of hepatotoxicity caused by antiretroviral therapy in patients with HIV [41]. Concomitant drugs can modulate the metabolism of other drugs through induction, inhibition or substrate completion of CYP450 reactions and hepatic transporter systems, which could affect their hepatotoxicity potential.
Another example is a non-alcoholic fatty liver disease (NAFLD) that is highly prevalent in western countries, and hence it frequently co-exists with DILI [42]. Individuals with pre-existing liver disease, which mainly included hepatitis C and NAFLD, were found to have higher mortality from DILI (16% versus 5.2%) in a recent update of the DILIN database [16].
Schistosomiasis is a parasitic disease that affects over 218 million people worldwide, mostly in Africa, South America and the Caribbean. The parasite's eggs are located within portal tracts inducing an extensive typical pipe stem fibrosis and portal hypertension [43]. Schistosoma mansoni is also commonly seen in patients co-infected with HIV in endemic areas. A cross-sectional study in Tanzania showed a strong association between underlying schistosomiasis and hepatotoxicity in HIV-infected patients taking antiretroviral therapy [44]. These authors observed that hepatotoxicity was three times more likely to occur in schistosomiasis-HIV coinfected patients than those carrying HIV infection alone [44].
Points to highlight
- •
Older age may be considered a contributing factor determining the susceptibility to DILI, secondary to particular drugs, and contributing to the phenotype of DILI.
- •
According to the EASL guidelines of DILI ethnicity should be considered a risk factor for liver toxicity.
- •
Well-designed prospective studies comparing DILI risk in different ethnics groups should be one of the main goals for the future.
- •
Although it has not yet been definitively confirmed, evidence suggests that schistosomiasis should be considered as a potential risk factor for DILI in HIV patients
- •
It could be interesting to validate these results in a prospective study including non-HIV patients carrying schistosomiasis.
DILI may mimic any liver injury in both histological and clinical patterns. Therefore, some rare clinical and pathological phenotypes like acute DILI becoming chronic, chronic hepatitis (usually with autoimmunity features), fibrosis, cirrhosis, vascular changes and ductopenic disease can be occasionally observed.
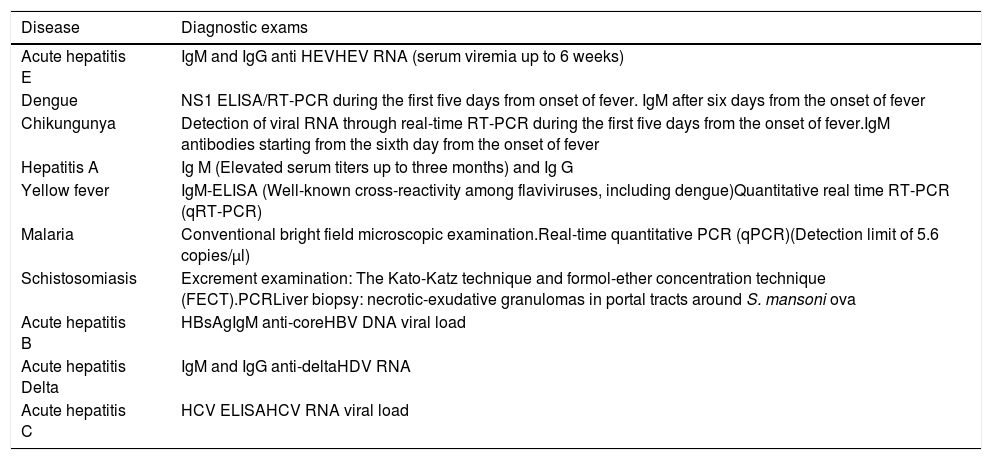
6.1Acute hepatitisSeveral differential diagnoses should be taken into account in LA before the diagnosis of acute liver toxicity be established (see also “Differential diagnosis in patients from Latin America”). Due to the existence of several endemic areas for schistosomiasis, yellow fever, dengue, chikungunya, zica, hepatitis E, hepatitis C, hepatitis B, with or without delta coinfection in some Latin American countries, clinicians should be aware on this issue when performing the work up assessment for DILI (Table 2).
Main infectious diaseases mimicking DILI to be ruled out in several Latin American countries.
| Disease | Diagnostic exams |
|---|---|
| Acute hepatitis E | IgM and IgG anti HEVHEV RNA (serum viremia up to 6 weeks) |
| Dengue | NS1 ELISA/RT-PCR during the first five days from onset of fever. IgM after six days from the onset of fever |
| Chikungunya | Detection of viral RNA through real-time RT-PCR during the first five days from the onset of fever.IgM antibodies starting from the sixth day from the onset of fever |
| Hepatitis A | Ig M (Elevated serum titers up to three months) and Ig G |
| Yellow fever | IgM-ELISA (Well-known cross-reactivity among flaviviruses, including dengue)Quantitative real time RT-PCR (qRT-PCR) |
| Malaria | Conventional bright field microscopic examination.Real-time quantitative PCR (qPCR)(Detection limit of 5.6 copies/μl) |
| Schistosomiasis | Excrement examination: The Kato-Katz technique and formol-ether concentration technique (FECT).PCRLiver biopsy: necrotic-exudative granulomas in portal tracts around S. mansoni ova |
| Acute hepatitis B | HBsAgIgM anti-coreHBV DNA viral load |
| Acute hepatitis Delta | IgM and IgG anti-deltaHDV RNA |
| Acute hepatitis C | HCV ELISAHCV RNA viral load |
IgM, Immunoglobulin M; IgG, Immunoglobulin G; HEV, Hepatitis E virus; HEV RNA, Hepatitis E virus ribonucleic acid; HCV, Hepatitis C virus ribonucleic acid; NS1 ELISA/RT PCR, Non structural protein-1 Enzime immuno assay/Reverse transcriptase-polymerase chain reaction; qRT-PCR, Quantitative real-time PCR; HBsAg, Hepatitis B virus surface antigen; IgM anti-core, Hepatitis B core IgM antibody; IgG anti-core, Hepatitis B core IgG antibody, HBV DNA viral load, Hepatitis B virus desoxiribonucleic acid viral load; HDV RNA, Hepatitis delta virus ribonucleica cid.
Chronicity in DILI is a currently evolving issue. A comprehensive literature review on drug-induced chronic liver injury shows that chronic DILI is much more common (18%) than previously thought [45]. In contrast with the classical cut-off point of 6 months of the persistence of liver enzyme abnormalities used to define chronicity in acute hepatitis, a recent study observed that 92% of patients resolved their liver damage ≤ one year after DILI recognition, indicating that 12 instead of 6 months should be considered to establish chronicity in DILI [46].
6.3Chronic hepatitisDrug-induced chronic hepatitis is a rare diagnosis in clinical practice and may show similar pathological features to those associated with hepatotropic viruses. In these cases, the diagnosis must be carried out in the setting of patients with a long history of drugs or herbs consumption, in the absence of both viral and autoimmune markers. The offending drug's withdrawal should be followed by normalization of liver enzymes to reinforce the DILI diagnosis. Nitrofurantoin, statins, minocycline and checkpoint inhibitors are only some examples of this clinical pattern [47,48].
Sometimes, drug-induced chronic liver disease diagnosis may be challenging, as it cannot be differentiated from classical autoimmune hepatitis (AIH). However, if prolonged or chronic hepatitis appears needing corticosteroids therapy, drug-induced AIH diagnosis should only be sustained if the withdrawal of steroid treatment is followed by the absence of biochemical reactivation of transaminases [48].
6.4Vascular diseaseDrugs may also cause damages at different vascular tree levels, mainly on both sinusoidal endothelium and central vein of the lobules. Toxic metabolites of some drugs may also impact on hepatic stellate and epithelial cells. Alcohol and vitamin A are two conspicuous examples of this condition [3].
Previously known as “veno-occlusive disease”, sinusoidal obstruction syndrome (SOS) is the most conspicuous vascular pattern characterized by the non-thrombotic obstruction of small veins, which display a deposit of myxoid matrix mixed with scarce inflammatory cells, within a condition of haemorrhagic necrosis of centrilobular hepatocytes. This rare DILI phenotype should be suspected in patients with bone marrow transplantation receiving high doses of busulfan monotherapy or associated with cyclophosphamide [49].
6.5Ductal damage and ductopeniaDuctal damage can be aggressive, destructive and progressive enough to be perpetuated over time. There are more than 70 drugs reported to induce ductal damage and at least three described mechanisms by which these compounds may induce ductopenia: (1) direct cholangiocyte attack by drugs, or their toxic metabolites, once they have been secreted into the bile, (2) immune-mediated, drug-induced cholangiocyte attack, (3) Sustained exposure to toxic bile salts that break the protective defenses of the biliary epithelium [50].
It is interesting to know that this event is not dose-dependent and usually has a delayed onset regarding clinical presentation and outcome. It also can be severe enough to evolve to biliary cirrhosis needing a liver transplant.
Clinicians should suspect this scenario when an abnormal ALP level and GGT persists even though the patient normalized transaminases and bilirubin. After a year of persistently high levels of ALP, a liver biopsy should be proposed. Of note, immunostaining using cytokeratin 7 may evidence the presence or absence of interlobar bile duct to support or ruled out a diagnosis of vanishing bile duct syndrome [51].
6.6Fibrosis and cirrhosisSinusoidal fibrosis, which is characterized by the deposition of fine collagen fibres in the sinusoids, has been generally associated with hypervitaminosis A and methotrexate (MTX). Hypervitaminosis A is characterized by marked hypertrophy of stellate cells within the space of Disse, and lipid vacuoles localized between the endothelium and the hepatocyte's plates [52].
Interestingly, MTX that can induce acute liver injury at high intravenous doses can also lead to drug-induced hepatic steatosis and chronic fibrosis, which may progress to cirrhosis in chronic therapy [53].
The occurrence of end-stage liver disease due to MTX toxicity is a rare clinical presentation. Reinforcing this statement, an analysis of the OTPN/UNOS database, found that only 0.07% of patients listed for liver transplantation in the US had MTX-induced liver disease [54].
Points to highlight
- •
Unusual phenotypes of DILI tend to mimic other etiologies of liver diseases in clinical practice. Hepatotoxicity should always be suspected when a specific diagnosis other than DILI has not been fully settled.
- •
There is still no consensus on how long and what schedule of immunosuppressive drugs should be prescribed when we faced with drug-induced AIH.
- •
No treatment has yet been shown to benefit ductal damage and drug-induced ductopenia.
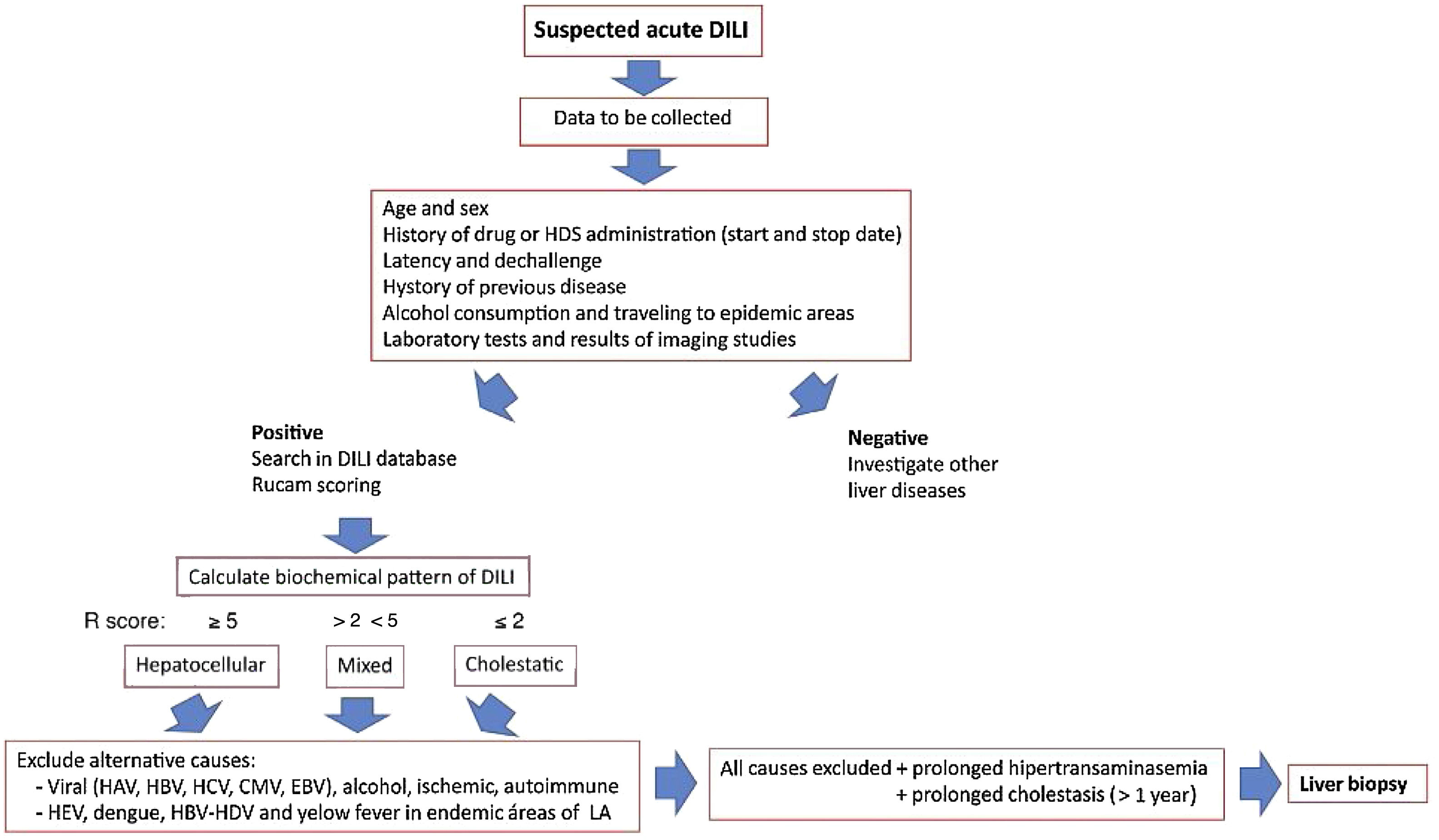
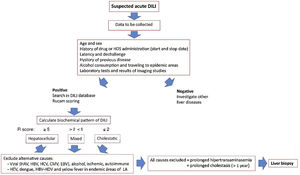
Since the onset of DILI varies broadly ranging from asymptomatic hypertransaminasemia to acute liver failure, the clinicians must follow a careful liver toxicity assessment, taking into account several important factors, such as a history of the suspected drug, time to DILI clinical onset, and preexisting liver diseases, in order to confidently rule out other diagnostic possibilities. An approach for diagnosis and early management of DILI is shown in Fig. 2.
Approach for diagnosis and early management of DILI. Bichemical pattern of DILI was defined as hepatocellular when patients presented a 5-fold or higher rise in alanine aminotransferase (ALT) alone or when the ratio of serum activity (activity is expressed as a multiple of the upper limit of normality [ULN]) of ALT to alkaline phosphatase (ALP)) was 5 or more. Liver injury was defined as cholestatic when a 2-fold or higher rise in ALP alone or when a ratio of serum activity of ALT to ALP of 2 or lower was observed. When the ratio of the serum activity of ALT to ALP was between 2 and 5, liver injury was termed mixed.
The CIOMS scale (also referred to as the Roussel Uclaf Causality Assessment Method, RUCAM) is still the most commonly used DILI causality assessment scale [55]. While being an excellent checklist, highlighting essential features for a DILI diagnosis, the CIOMS/RUCAM scale also has limitations. These include the absence of clear instructions to answer the scale poses, a low diagnostic capability when multiple drugs with the same temporal relationship are present and when acute liver failure is one of the potential DILI diagnosis. This scaling method is also used for herb-induced liver injury (HILI) and intoxications to follow a rigorous guide and exclude alternative causes.
It is also expected to improve RUCAM, adding biomarkers or other criteria provided that the validation process replaces expert opinion by robust standards such as those used for the original method [3,56].
Points to highlight
- •
Training on CIOMS/RUCAM should be encouraged to improve the epidemiological studies, case discussion in clinical practice, pharmacovigilance organisms or regulatory agencies.
- •
An expert group of specialists is working to improve this causality scale, including biomarkers and other criteria, to replace expert opinion by a more robust tool for DILI diagnosis.
Suspicion of DILI is a potential indication for liver biopsy. Although liver biopsy interpretation does not replace R-value for classification purposes, occasionally, it may confirm the biochemical pattern. The hepatocellular pattern of liver injury (R≥5) shows more severe inflammation, necrosis and apoptosis, while patients with the cholestatic type of liver injury (R≤2) more often is characterized by bile plugs, ductal damage and ductopenia [51].
Moreover, a liver biopsy may help suspect DILI diagnosis, when dealing with specific histological features of this injury (e.g., elevated eosinophil count, low-grade lobular hepatitis associated with granulomas, zonal necrosis, microvesicular steatosis, centrilobular hepatocellular injury, and hepato-canalicular cholestasis associated with mild liver disease). Furthermore, individual agents may cause several specific patterns of hepatotoxicity, and the pathologist may identify these histological scenarios so that clinicians may suspect the potential culprit agent of liver toxicity. This situation can especially be useful regarding DILI occurring during polypharmacy in elderly patients [57,58].
Interestingly, biological agent-induced hepatotoxicity is a new chapter that we are still learning. It is characterized by drugs linked to a different mechanism of liver injury triggered by immune dysregulation.
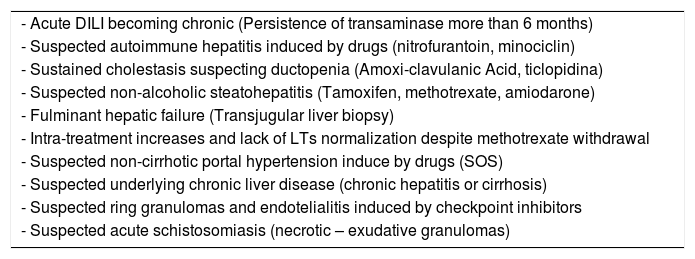
Immune checkpoint inhibitors-induced liver damage may present distinctive histological features characterized by ring granuloma and endothelialitis in the context of immune-mediated hepatitis that make liver biopsy an important diagnostic option [59]. Despite that around of 50% of these cases responds to corticosteroid therapy, one of the most current concerning dilemmas and controversies surrounding this issue is the indication, timing, dosage, and duration of steroid treatment [3]. Potential indications of liver biopsy are shown in Table 3.
Suggested indications of liver biopsy in acute and chronic DILI.
| - Acute DILI becoming chronic (Persistence of transaminase more than 6 months) |
| - Suspected autoimmune hepatitis induced by drugs (nitrofurantoin, minociclin) |
| - Sustained cholestasis suspecting ductopenia (Amoxi-clavulanic Acid, ticlopidina) |
| - Suspected non-alcoholic steatohepatitis (Tamoxifen, methotrexate, amiodarone) |
| - Fulminant hepatic failure (Transjugular liver biopsy) |
| - Intra-treatment increases and lack of LTs normalization despite methotrexate withdrawal |
| - Suspected non-cirrhotic portal hypertension induce by drugs (SOS) |
| - Suspected underlying chronic liver disease (chronic hepatitis or cirrhosis) |
| - Suspected ring granulomas and endotelialitis induced by checkpoint inhibitors |
| - Suspected acute schistosomiasis (necrotic – exudative granulomas) |
AIH, Auotinmmune Hepatitis; LTs, Liver Tests; SOS, sinusoidal Obstructive Syndrome.
Points to highlight
- •
In general, DILI diagnosis does not require a liver biopsy, but it can help to confirm a clinical suspicion of DILI, by excluding other causes.
- •
Other patterns as AIH induced by drugs, steatohepatitis associated with MTX and SOS linked to intake of oncologic drugs can also be suspected on liver histology
- •
In patients suffering prolonged cholestasis, liver biopsy is the best tool for diagnosis of bile duct damage and vanishing bile duct syndrome induced by drugs
- •
A liver biopsy may also be useful in those patients suspected to have chronic hepatitis induced by drugs medicaments linked to an absence of serological markers
- •
Suspected drug-induced granulomatous hepatitis needs liver histology evaluation to approach this diagnosis or to rule out other causes of liver granulomata.
The existing clinical scoring systems have a limited predictive value associated with certain inherent deficiencies, so the diagnosis of DILI in clinical practice would benefit from novel biomarkers. The advent of omics technologies offers a new approach to provide valuable information on various mechanistic-based biomarker candidates, including glutamate dehydrogenase, high-mobility group box 1 protein, keratin-18 and microRNAs (mainly miR-122), which are being tested as non-invasive DILI biomarkers. It could represent a significant advance in the management of hepatotoxicity by increasing sensitivity and specificity in DILI diagnosis [60].
MicroRNA-122 (miR-122) is a hepatocyte-specific miRNA that is elevated in the patient's plasma within hours of an APAP overdose [61]. High mobility group box-1 (HMGB1) and keratin-18, have been shown to predict the subsequent onset of liver injury early before ALT elevation [62]. Macrophage colony-stimulating factor receptor 1 (MCSFR1) markers of immune activation where both MCSFR1 and the biomarker osteopontin were elevated in serum in 31 patients associated with DILI fulfilled Hy's Law criteria compared with 70 patients with DILI who did not fulfil these criteria [1].
Serum bile acid as glycodeoxycholic acid (GDCA) has been shown to have prognostic value in predicting the outcome of acute liver failure induced by APAP. It has been shown that GDCA levels were higher in non-surviving patients with ALF [63]. Interestingly, circulating bile acid (BA) profiles are currently being evaluated as biomarkers for hepatotoxicity [64,65].
Points to highlight
- •
Predictive value from several clinical scoring systems is still limited. Thus, the most significant future diagnosis benefit would be achieved by combining the diagnostic scales and biomarkers.
- •
The pharmaceutical companies have shown interest in miRNA profiling. Developing PCR-based miRNA panels appears to be a promising approach.
DILI often represents a challenge for the physician in clinical practice where a meticulous data collection is crucial for diagnosis. As previously discussed, almost any clinical pattern and histological features of liver disease can be due to DILI, ranging from a mild elevation of liver enzymes to acute liver failure. DILI may trigger several liver damage forms, including chronic hepatitis, chronic cholestasis, liver cirrhosis, obstructive sinusoidal syndrome, non-alcoholic steatohepatitis, and even benign and malignant tumours. This complex universe of variables that the clinician should keep in mind when studying a patient with a potential DILI impacts the final diagnosis's accuracy.
Interestingly, a recent review article by Teschke et al. [66] showed that DILI reports often did not provide all the information needed to determine the cause of the liver injury. Reviewers and editors did not pay sufficient attention to the completeness of the submitted case report for publication.
We have to keep in mind that many areas worldwide present different incidences and prevalence of the liver disease, representing a real challenging background for patients with suspected DILI. After ruling out acute viral hepatitis using conventional diagnostic tests, the clinician should know whether the liver disease is more likely linked to DILI or a flare triggered by a pre-existing chronic liver disease.
The classical differential diagnosis for acute hepatocellular injury includes viruses like hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis E virus (HEV), cytomegalovirus (CMV) and Epstein-Barr (EBV), autoimmune hepatitis, ischaemic liver injury (heart failure, hypotension, hyperthermia, liver hypoxia), and Wilson disease. Anti-HCV antibody may be initially negative and acute hepatitis C should be excluded by HCV RNA testing [3,67].
Latin America shows a variable HBV and HCV prevalence rates ranging from 0.6 to 8% [68,69], but when discussing between DILI and viral hepatitis, the most important differential diagnosis to rule out is hepatitis E virus (HEV). Studies on prevalence rates in the human population from Latin American countries have shown a moderate endemicity to HEV, ranging from 1 to 10%, although it might be underestimated, due to former immunoassays insensitivity [70].
Because of the low reliability of serological markers to detect hepatitis E, and since HEV viraemia can be prolonged more than five weeks, acute HEV infection should be ruled out by detecting HEV RNA assay [71] (Table 2).
Two studies have evaluated the presence of anti-HEV antibodies and serum HEV RNA in cohorts of patients with suspected DILI retrospectively. In a small cohort of 28 patients with suspected DILI and sera available from the presentation time, HEV was detected as a final diagnosis in 6 (21%) [72]. On the other hand, the second study published by DILIN group showed acute hepatitis E in 9 out of 318 (3%) suspected DILI cases [73].
In both studies, patients with hepatitis E were significantly more likely to be male and older than those associated with DILI. In the DILIN study, serum bilirubin, liver enzymes levels and R values, were not significantly different between HEV and DILI patients, emphasizing the importance of developing an accurate assessment for ruling out HEV.
Vector-borne diseases account for more than 17% of all infectious diseases. In LA, dengue and chikungunya constitute a potential epidemiological risk, due to the recent increase in cases, complications, and severity [74]. They are prevalent systemic viral diseases in several Latin-American countries that can be associated with liver compromise and, sometimes, they mimic acute DILI. Dengue is an endemic disease in Argentina, Brasil, Chile, Colombia, Mexico, Nicaragua, Peru, Paraguay, Puerto Rico, Dominican Republic, and Uruguay. It is usually linked to a self-limiting disease, but mild reactive hepatitis commonly occurs. Despite the high frequency of liver compromise characterized by anicteric hypertransaminasemia, it is rarely associated with severe acute hepatitis evolving to liver failure. The full disease is frequently preceded by a flu-like syndrome characterized by high fever and intense myalgia. Specific IgM tends to induce a high percentage of false-positive with other arboviruses, whereas PCR becomes the most useful diagnostic tool [75].
Liver involvement associated with chikungunya is uncommon and occurs less often than with dengue. Severe forms of liver injury and acute liver failure have not been reported [74,76].
Regarding yellow fever and according to data from the Panamerican Health Organization (OPS), six countries and territories in LA reported confirmed cases between January 2017 and November 2018, namely Bolivia, Brazil, Colombia, Ecuador, French Guyana, and Peru [77]. Although yellow fever has a dengue-like onset, it is more frequently associated with severe liver damage forms evolving to liver failure as the most important cause of death in these patients. Contrarily to what has been described for dengue disease, serologic analysis of IgM has a high diagnostic value, whereas PCR is used only in doubtful cases [78].
Acute schistosomiasis is a systemic hypersensitivity reaction against the migrating schistosomula and eggs. Serology may help diagnose, but the finding of necrotic-exudative granulomata in a liver biopsy specimen is the pathognomonic hallmark of acute liver disease [79].
The highest prevalence of hepatitis B and delta viruses (HDV) (up to 8%) is found in the Western Amazon Basin, including Brazil, Peru, Ecuador, Venezuela, and Colombia [80]. Diagnosis of HDV infection is based on clinical, biochemical, serological, histopathological, and virological criteria. Immunoglobulin G anti-HDV is an antibody that indicates contact with the virus, and immunoglobulin M anti-HDV denotes active acute or reactivated chronic infection [81] (Table 2).
Points to highlight
- •
Hepatitis E is one of the most confusing differential diagnoses of DILI when clinicians suspect hepatotoxicity, and it should always be taken into account as the first line of differential diagnosis when finding clinical hepatocellular and mixed phenotypes.
- •
It is important to highlight that antibodies anti HCV may be initially negative, and acute hepatitis C should be excluded by HCV RNA determination
- •
Dengue and yellow fever should be added to the differential diagnosis of DILI in several Latin-American endemic regions, mainly when severe or fulminant liver diseases have been the main phenotype at presentation.
- •
Yellow fever is usually associated with more severe liver disease than dengue; in this case, serologic analysis of IgM has a high diagnostic value, whereas PCR is used only in doubtful cases.
- •
Acute schistosomiasis is characterized by a clinical hypersensitivity presentation that can sometimes mimic mixed DILI, since it may induce granulomatous hepatitis confirmed by liver biopsy.
- •
Hepatitis B, with or without delta virus, should be taken into account in high endemic areas for both viruses and can be quickly ruled out by using serological and virological determinations.
The treatment of idiosyncratic DILI is still based mainly on supportive care. Suspicion and immediate discontinuation of all drugs that the patient has been taking are crucial to prevent persistent damage. An algorithm for the management of DILI is shown in Fig. 2.
Jaundiced patients with acute hepatocellular injury may need to be referred to a specialized liver unit because of the increased risk of liver failure progression [82].
One randomized controlled trial reported of N-acetylcysteine (NAC) therapy is useful not only in paracetamol hepatotoxicity but also in acute liver failure linked to other idiosyncratic DILI causes [83]. This study also demonstrated that NAC was useful when indicated to patients with fulminant hepatitis who presented hepatic encephalopathy grade I and II.
Corticosteroids can be useful for DILI associated with autoimmune or systemic hypersensitivity features. It should be considered in cases with histological-proven DILI-associated autoimmune hepatitis. In most patients, corticosteroids can be reduced and stopped after months [84]. Unfortunately, it does not improve patients’ overall or spontaneous survival with non-autoimmune acute drug-induced liver failure. Conversely, it may increase the rate of infectious complications following liver transplantation [85].
Of note, autoimmune hepatitis triggered by checkpoints inhibitors (ICI) and anti–TNF drugs deserves discussion as a separate topic [86]. The EASL guidelines suggest that the decision of steroid therapy in severely ill DILI patients should follow a multidisciplinary approach based on clinical and histological assessments [3]. On the other hand, oral prednisone and intravenous methylprednisone are recommended for grade 2–4 immune-related ICI hepatitis, according to the Guidelines from the American Cancer Association [87]. French authors have recently proposed a different point of view suggesting that after adopting an individualized approach; almost half of the patients with DILI grade 3 or 4 spontaneously improved liver disease without corticosteroid therapy [88].
Silymarin and glycyrrhizin have been used to treat DILI for decades, but success remains anecdotal. Bile acid washout regimens using cholestyramine appear to be more evidence-based, particularly for leflunomide toxicity [89].
Anecdotal small series suggests that ursodeoxycholic acid (UDCA) treatment may be beneficial in some forms of drug-induced cholestasis. UDCA protects hepatocytes and cholangiocytes by replacing endogenous and cytotoxic bile salts. UDCA also induces functional transporters’ expression at the transcriptional and post-transcriptional level and enhances bile flow [90].
A randomized trial of L-carnitine in children with severe liver injury associated with valproate therapy showed a dramatic effect on survival in patients treated with this compound compared with those patients receiving placebo [91].
For drug-induced acute liver failure, the use of liver support systems is still investigational in the United States, and emergency liver transplant remains limited by its availability [92]. Primary prevention appears to be the key to avoiding DILI and the need for acute treatment.
Points to highlight
- •
A detailed investigation of potential drugs causing DILI should be performed. Any potential hepatotoxic drug or substance should be initially removed.
- •
N-acetylcysteine therapy should be taken into account for treating ALF induced by other drugs than paracetamol.
- •
Cholestyramine appears to be more evidenced-based, in particular for leflunomide toxicity.
- •
Administration of L-carnitine showed benefits on survival in children with severe liver injury associated with valproate therapy.
- •
Attempting a UDCA course in prolonged cholestasis induced by drugs might be beneficial in a low number of cases improving both pruritus and biochemical parameters.
The outcome in DILI is foremost benign with complete recovery in most instances, but it also can lead to hospitalization in the short term, life-threatening liver failure resulting in death, or need for LT. An overall 5–10% of the cases do not survive or require a liver transplant, being the fatality rate even higher in the hepatocellular pattern when associated with jaundice [3,18,93].
High bilirubin levels, female sex, and a marked rise of AST and AST/ALT ratio >1.5 at DILI recognition also predicts a worse prognosis [3,19,94].
Initial DILI assessment should also include coagulation parameters. Elevated international normalized ratio (INR) values, suggest impending liver failure and should prompt referral to a liver transplant unit. A recently described new Hy's law (nR Hy's law) algorithm was validated in patients included in both Spanish and LATNDILI registries. It was defined as a TBL higher than 2×ULN and hepatocellular pattern defined as a new ratio value of five or greater (nR=ALT or AST, whichever produced the highest R-ratio). This score showed better discrimination accuracy than traditional Hy's law with similar sensitivity (≈90%) but significantly higher specificity (63% vs. 43%) [94]. These authors concluded that patients with AST over 17.3×ULN, TBL over 6.6×ULN showed an increased risk of evolving to ALF or LT. Those patients with AST <17.3×ULN but AST/ALT ratio greater than 1.5 have also increased risk of the detrimental outcome. This algorithm identified patients at high-risk ALF development with 80% sensitivity, 82% specificity and AUROC 0.80.
Point to highlight
- •
Although the DILI prognosis is usually benign, clinicians should be aware that a small percentage of cases may progress to acute liver failure. Prolonged forms of DILI, both hepatocellular and cholestatic that do not resolve within the first year from starting symptoms present a high probability to evolve to chronic liver disease.
An adequate interval for monitoring has not been yet well established, and a monthly biochemical control has not been proven to be effective [3].
Probably, the monitoring of risk of hepatotoxicity with anti-TB has been the best-studied scenario as it has been proposed by the American Thoracic Association [95]. They have proposed monitoring those patients linked to DILI risk factors like chronic alcoholism, concomitant hepatotoxic drugs, underlying liver disease, pregnancy and previous DILI induced by isoniazid.
It has also been suggested that a weekly control of ALT and withdrawal of drug therapy should be carried out if ALT >3×ULN associated with symptoms or total bilirubin increase, or when ALT >5×ULN in the absence of symptoms [96].
Follow-up in DILI patients must include routine liver biochemistry until complete normalization. Persistently elevated TBL and ALP 30 to 60 days after DILI recognition are reasonably predictive of chronic outcome [97]. Cholestatic cirrhosis and ductopenia, which is poorly responsive to UDCA, may develop as a prolonged DILI sequel. A liver biopsy may be useful in this group of patients presenting elevations of ALP and GGT levels with suspicion of ductopenia or granulomas [98,99]. In addition to compliance issues, idiosyncratic DILI can have a long latency before manifesting clinical DILI [3].
Points to highlight
- •
Despite that monitoring interval of DILI has not yet been established, it would be important to emphasize that when an anti-TB scheme is prescribed, close monitoring should be carried out.
- •
Drugs using immunoalergic mechanisms of liver damage should not be closely monitored (e.g. phenytoin, carbamazepine).
As it has been previously described, causality scales have been developed to guide the clinician in diagnosis, and both several database and registries are available for reference and reporting DILI cases [55]. The management and assessment of the DILI represents a real challenge in clinical practice that has led to the creation of numerous online hepatotoxicity resources for further guidance [100–102].
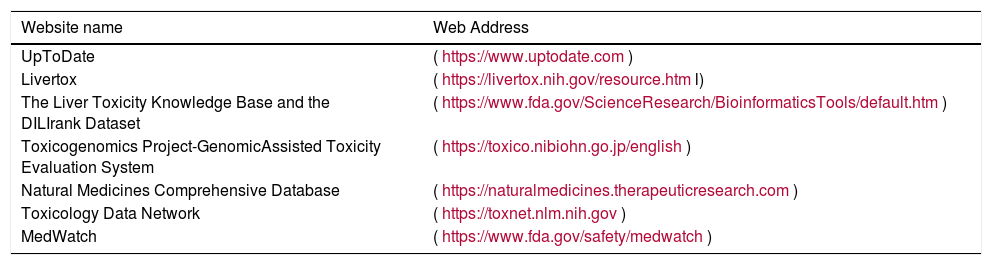
The most important website sources for searching drugs and herbs associated with potential DILI are shown in Table 4. One of the most conspicuous examples is Livertox, a free web site that provides concise, unbiased, accurate, and easily accessed drug records. It includes information on the details of hepatotoxicity caused by both prescription and nonprescription medications and HDSs. LiverTox currently hosts data on 1124 different compounds, including 23,000 annotated references, and 400 case descriptions [103].
Physician websites for hepatotoxicity research.
| Website name | Web Address |
|---|---|
| UpToDate | (https://www.uptodate.com) |
| Livertox | (https://livertox.nih.gov/resource.html) |
| The Liver Toxicity Knowledge Base and the DILIrank Dataset | (https://www.fda.gov/ScienceResearch/BioinformaticsTools/default.htm) |
| Toxicogenomics Project-GenomicAssisted Toxicity Evaluation System | (https://toxico.nibiohn.go.jp/english) |
| Natural Medicines Comprehensive Database | (https://naturalmedicines.therapeuticresearch.com) |
| Toxicology Data Network | (https://toxnet.nlm.nih.gov) |
| MedWatch | (https://www.fda.gov/safety/medwatch) |
Point to highlight
- •
The clinician should be aware of the relevant information provided for several web pages for searching potential implicated drug in DILI. Some of them encompass not only complete drug information but also diagnostic scales and guidance on drug-drug interactions.
The LATINDILI registry is still a young network, but it is emerging as a solid working group, including more than 350 DILI cases. It is positioning in the specialized international community due to its rigorous performance and scientific validity. With the experience gathered throughout these years, we believe that the primary attention should be on education and training to improve LA's diagnostic skills.
- (1)
To characterize the signature of the most frequently involved drugs in DILI, the host and drug risk factor modifiers, and outcome in this population.
- (2)
To obtain robust results coming from well-designed studies on the prevalence and incidence of DILI.
- (3)
To identify all culprit herbal and supplements in different Latin American countries capable of inducing liver toxicity.
- (4)
To increase physician awareness on both the phenotypes inducing DILI/HILI in LA and LATINDILI registry's existence for submitting cases linked to liver toxicity.
- (5)
To foster collaborative activities with the regulatory agencies to detect safety signals that can be managed comprehensively. Latin American countries need clearer rules for ensuring liver safety of drugs and HDS.
- (6)
To participate in studies for the qualification of biomarkers and other international joint activities to better understand DILI.
- (7)
To participate in collaborative pharmacogenetic studies collecting biological samples to identify DILI impact according to different Latin American ethnicities.
- (8)
To participate in collaborative and multicenter studies with other DILI registries defining algorithms that reliably predict DILI, host factors and mechanistic ways.
If we could summarize all of the above proposals into a single goal for the future, we should emphasize that we need a spark of enthusiasm from physicians and researchers cooperating on time to better detect and understand DILI in our region.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to thanks Professors Raul J. Andrade Bellido and Maria Isabel Lucena Gonzalez for their permanent support and constant stimulus since the beginning of our LATINDILI Registry.
The authors also wish to thank the valuable collaboration of Professors Ramón Bataller and Raúl J. Andrade for reviewing this manuscript.





 DILI. Bichemical pattern of
DILI. Bichemical pattern of 



