For the pathologist, the diagnosis of drug induced liver injury (DILI) is challenging, because histopathological features mimic all primary hepatic and biliary diseases, lacking changes that are specific for DILI. Therefore, in any patient of suspected DILI who underwent liver biopsy, the pathologist will assure the clinician that the observed hepatic changes are compatible with DILI, but this information is less helpful due to lack of specificity. Rather, the pathologist should assess liver biopsies blindly, without knowledge of prior treatment by drugs. This will result in a detailed description of the histological findings, associated with suggestions for potential causes of these hepatic changes. Then, it is up to the physician to reassess carefully the differential diagnoses, if not done before. At present, liver histology is of little impact establishing the diagnosis of DILI with the required degree of certainty, and this shortcoming also applies to herb induced liver injury (HILI). To reach at the correct diagnoses of DILI and HILI, clinical and structured causality assessments are therefore better approaches than liver histology results obtained through liver biopsy, an invasive procedure with a low complication rate.
Drug induced liver injury (DILI) is no uniform and therefore no concisely describable disease entity due to its multiple facets, since it bundles variable clinical manifestations caused by numerous drugs with different chemical structures.1,2 The heterogeneous appearance of DILI is a particular issue, since the diagnosis of DILI still is one of exclusion and not based on established diagnostic biomarkers. A similar heterogeneity applies to herb induced liver injury (HILI) caused by various herbs with multiple ingredients.3 As a diagnosis of exclusion, both DILI and HILI compete with some hundred primary liver diseases unrelated to drugs and herbs, which are to be excluded and require a thorough diagnostic work up.1–3 In initially assumed liver injury cases, the frequency of alternative causes is high,4,5 raising the question whether images data such as liver histology could be helpful establishing the diagnosis on a firm basis.
The present report addresses the question of liver histology as a useful tool in liver injury cases, since liver biopsy is an invasive procedure with rare risks of some associated complications.
Frequency of Liver Biopsy in Dili and Hili CasesIn one single DILI case series, a liver biopsy was an obligatory item for all patients to be included in a clinical study for diagnostic work up and a causality assessment algorithm.6 In HILI case series, an obligatory requirement for liver histology in the course of a diagnostic work up has not been described in any report.
In most DILI and HILI case series published within the past two decades, some but never all patients underwent liver biopsy, suggesting a facultative approach. In DILI case series, for instance, the percentages of patients with liver biopsy were 65%,7 62%,8 35%,9 23%,10 and 13%.11 For HILI case series, the corresponding figures were 70%,12 44%,13 42%,14 and 40%,15 as shown in some reports as examples. In studies consisting of both DILI and HILI cases, patients underwent liver biopsy in 13%.16 In other case series studies, results of liver biopsy were not available,17–19 or histological results were extracted from the medical charts without any further reported quantitative or qualitative details or evaluations.20 It appears that at present liver biopsy is a facultative rather than an obligatory measure for DILI and HILI case assessment.
In studies focussing on the natural course of acute idiosyncratic DILI, all patients underwent liver biopsy in the further course to chronic hepatotoxicity.21,22 This approach is primarily of academic interest to detail the natural course of the disease, but it lacks actual benefit for the treatment of the individual patient and outcome of the disease.
Positive Re-Exposure Test and Liver BiopsyA positive unintentional re-exposure test represents the highest level of diagnostic certainty achievable in cases of unpredictable liver injury. Consequently, this unintentional test is at present considered as a gold standard to firmly establish the diagnosis retrospectively,1,13 provided specific test criteria are fulfilled.13 In numerous DILI and HILI cases with a positive test result, however, a liver biopsy was in addition done subsequently to the unintentional reexposure, possibly to ascertain the diagnosis in these particular cases.10,13 More specifically, in a recent HILI study of 34 cases all with a reported positive reexposure test, 15 patients (44%) underwent a liver biopsy as a supposed additional diagnostic aid, including one patient biopsied trice in the course of the disease.13 The evaluation of these 15 cases with HILI plus a reported positive re-exposure indicated that the patients did not profit from the results of the liver biopsy, neither diagnostically nor therapeutically.13 Of note, the value of liver histology in establishing the diagnosis or contributing to causality considerations has not been validated and is open for discussion due to the unspecificity of histological results in DILI and HILI.23,24 Thus, the indication for a liver biopsy in cases with existing positive re-exposure results cannot be based on prior diagnostic uncertainty.
Liver Histology for Case CharacterizationAt earlier times when transaminases as well as liver specific serological and immunological parameters were not available for routine diagnostic purposes, liver histology was considered the gold standard to diagnose various liver diseases. Later on, individual liver diseases including DILI and HILI were described using laboratory results and possibly liver histology data. Based solely on laboratory criteria of ALT and ALP, DILI and HILI are now defined as hepatocellular, cholestatic or mixed type of liver injury, and it is clear that for this classification liver histology is not required any more.24,25 In addition, applying diagnostic causality assessment algorithms to verify the diagnosis, histological results again are no required items for the diagnosis26 but still available on a case-by-case basis.2,26,27
It is well recognized that previous liver histology findings substantially contributed to case characterization of DILI1,2 and HILI.1,24,27 In particular, under some conditions liver histology data may enable new disease characterization, recently described for instance in a case series with an established causality for newly recognized Greater Celandine hepatotoxicity.28 In 12/16 cases, liver histology was available and described with prevailing features of hepatitis, single or confluent liver cell necroses, inflammation, and rarely fibrosis and cholestasis, lacking a uniform picture.28 Liver histology was also used in case series for characterization of liver injury by other herbs such as kava29 and Polygonum multiflorum.15 In essence, liver histology did not provide any new information in addition to the prior laboratory disease classification; it was interesting for academic and clinical purposes but otherwise not helpful for the patients themselves.
Histological PatternA wide range of histopathological features of the liver has been described in numerous reports of DILI cases, as comprehensively outlined1,2,30 and documented by impressive pictures.30 Potentially hepatotoxic drugs can mimic virtually any form of some hundreds of liver diseases that are not associated with drug treatment and prevail as primary liver diseases in the general population.1,2 Consequently, results of liver histology per se are considered unspecific and do not allow DILI diagnosis with the required certainty.23
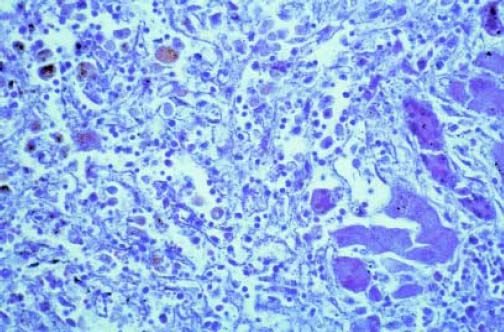
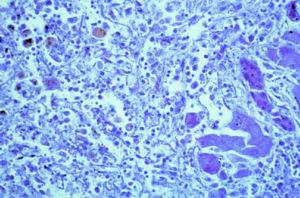
Acute hepatitis is the most common histological pattern of DILI.30 Its hallmarks are portal and parenchymal inflammation and hepatocellular injury, which may include liver cell necrosis. Inflammatory cells selectively consist of lymphocytes, plasmahistiocytic cells, and neutrophils.30 Liver cell necroses occur as spotty necroses affecting single cells or as confluent necroses involving groups of hepatocytes. When extensive, confluent necroses may cause acute liver failure lacking any normal liver cell, a further differentiation is not possible as shown in a case of Halothane hepatitis (Figure 1). Therefore, the predominant feature of acute hepatitis in DILI cases is inflammation alone or combined with liver cell necroses at various degrees, whereas liver cell necroses may also be observed in the absence of inflammatory cells as separate disease entity. Overall, the histological features of acute hepatitis as initially suspected DILI can be indistinguishable from other causes of acute hepatitis like acute viral hepatitis, initial presentation of autoimmune hepatitis, and Wilson disease.30 The presence of prominent eosinophilic infiltrates, granulomas, and sharply defined perivenular necrosis favours adverse drug reaction, but again, none of these features is DILI specific.
Acute drug induced cholestasis is another feature of DILI, with two different histological varieties; one showing bland cholestasis and the other one signs of an acute cholestatic hepatitis.30 The pure cholestatic type is characterized by bile plugs in liver cells and bile canaliculi, lacking signs of inflammation and hepatocellular injury including liver cell necrosis. This form is histologically indistinguishable from initial stages of obstructive biliary disease, systemic sepsis, cardiac failure, shock, postoperative cholestasis, benign recurrent intrahepatic cholestasis, and intrahepatic cholestasis of pregnancy. By contrast, the cholestatic hepatitis type provides histological signs of an acute hepatitis associated with cholestasis out of proportion to hepatocellular injury, with bile duct injury as an additional facultative item.30 The histological pattern of the acute drug induced cholestatic hepatitis may mimic obstructive biliary diseases and cholestatic forms of both autoimmune hepatitis and acute viral hepatitis, requiring thorough distinction. Drugs may also cause chronic cholestatic diseases including the vanishing bile duct syndrome with ductopenia, which all are to be differentiated from primary hepatobiliary diseases such as chronic obstructive disease, primary biliary cirrhosis, primary sclerosing cholangitis, and various chronic forms of intrahepatic cholestasis.
The histological pattern of DILI also includes autoimmune hepatitis, granulomatous hepatitis, steatohepatitis, chronic hepatitis, cirrhosis, peliosis, vascular injury including the sinusoidal obstruction syndrome (SOS), Ito cell lipidosis, adenomas, and malignant tumors.30 Again, these diseases are not specific for DILI and require clear differentiation.
In analogy to DILI,1,2,30 similar histological findings are reported for HILI caused by various herbs.24,27–29 Based on this similarity, there is no differentiation possible between DILI and HILI in patients comedicated with synthetic drugs and herbs.
Major concern relates to the bias in connection with the pathologist’s report, unless assessment is blinded. The physician commonly informs the pathologist about the drug and herbal medication of the patient under consideration at the time when the liver specimen obtained at biopsy is provided. The pathologist may offer final diagnoses such as drug or herb induced liver injury or liver injury compatible with or suggestive for drug or herbal use, leaving the impression of drug or herbal hepatotoxicity as a diagnosis confirmed by histology. The physician will present this case information to the regulatory agency; this spontaneous report will then be classified as proven herbal hepatotoxicity and considered as signal case. However, no objective, firm, and specific histological criteria to establish the diagnosis of drug and herb induced liver injury have been described in the literature. Therefore, the pathologist’s diagnosis of hepatotoxicity is circumstantial and the assumed diagnostic certainty is unwarranted. For causality assessment, the pathologist should provide the diagnosis of hepatitis and only suggest which of the multiple causes of hepatitis will fit best to the histological results. This will encourage the clinician to improve the search for alternative causes.
Risk of Liver BiopsyThe percutaneous liver biopsy is considered as a relatively safe procedure, especially in experienced hands.31 Patients with advanced liver insufficiency and liver cirrhosis present higher risks, with a mortality rate up to 19% in the first three months after biopsy.32 Overall, mortality directly related to the procedure is a rare event, varying from 0.01 to 0.1%. The main cause of death after liver biopsy is intraperitoneal hemorhage, with 0.03 to 0.07% incidence.31,32 In a recent study there were no deaths, major complications (other than pain) related to the procedure were observed in 7/1,955 cases, the complication rate thereby was 0.36%: 5 punctures where bile was aspirated, 1 pneumothorax and 1 hemoperi-toneum, which required surgery.31 Therefore, risks and benefits of a liver biopsy have to be weighted against each other, considering also the costs associated with liver biopsy and histological assessment.
Change of DiagnosisThere is little information that liver histology alone changed the diagnosis in initially assumed and otherwise carefully evaluated DILI and HILI cases. In one study with 77 DILI cases, liver biopsy performed in 10 patients (19.9%) showed findings that were compatible with DILI and in no case did the biopsy change the clinical assessment.11 Histological results also failed to change the diagnosis in HILI cases of one study.13 In 2 cases lacking an initial thorough clinical assessment, histology showed giant cell hepatitis and disproved the prior HILI diagnosis.24 There was no resulting consequence with respect to therapy.
Overall, a patient with suspected liver injury requires a sophisticated clinical assessment and causality evaluation by a liver specific algorithm such as the scale of the Council for International Organizations of Medical Sciences (CIOMS).26 If available, results of an unintentional re-exposure test can establish the diagnosis, provided required criteria are fulfilled.13,33 A liver biopsy in hepatotoxicity cases is only warranted when diagnostic uncertainty exists and alternative causes have thoroughly been ruled out before by careful evaluation of the medical history and by non invasive means like appropriate laboratory tests, immunological parameters, and images methods. A liver biopsy should be considered if liver values fail to decrease despite cessation of the assumed causative drug. In rare instances, liver histology may reveal a seronegative autoimmune hepatitis or an immune-allergic DILI; both conditions are possible candidates for a corticosteroid therapy.
On a case-by-case basis the decision has to be made whether the patient will benefit from histological results, keeping in mind that histology rarely provides specific and new diagnostic clues. Certainly, liver biopsy can confirm the biochemical classification, but this is of no benefit for the patient. There is general agreement that DILI and HILI histology is often non-specific, adds little to the accuracy of the diagnosis, and can mimic other primary acute and chronic liver diseases. Whether liver histology is useful for grading severity of acute injury or for assessing a chronic course of the disease should be decided on a case-by-case basis, considering the rare potential benefit for the individual patient.
Accepted for publication but still lacking copyediting and proofreading, an actual report of the Drug Induced-Liver Injury Network (DILIN) described and discussed hepatic histological findings in suspected DILI of 249 patients when liver biopsies were considered clinically necessary.34 Assessment was not blinded since the pathologist knew that DILI cases were to be evaluated that had been derived from DILIN. Cholangiolar cholestasis has been described as a characteristic finding in sepsis and may be acting as a marker for this comorbidity.34 However, systemic sepsis may be a missed diagnosis in initially assumed DILI4,10,17,21,35 and is certainly is not a histological but a clinical diagnosis not requiring liver biopsy. There is also the vague information that the criteria used to perform a biopsy may have varied among the eight enrolling centers, but details for specific indications were not provided. As explicitly mentioned, this study was not designed to address the diagnostic utility of a liver biopsy in DILI. The authors also point out that although biopsy may be a useful diagnostic and management tool in DILI, is was not possible to delineate specific advantages in the context of the current DILIN study. Indeed, in none of the 249 cases was there any change of the DILI diagnosis based on histological findings,34 not supporting the view that liver biopsy is a useful diagnostic and management tool in DILI. On the contrary, this study clearly provides evidence that liver biopsy was an unnecessary invasive procedure and of no benefit, at least for the 249 DILI patients analyzed in the actual study. This is the most important conclusion of this recent study to be drawn for future DILI cases.
ConclusionLiver histology is commonly of little impact establishing the diagnosis of DILI, and this shortcoming also applies to HILI. To reach at the correct diagnosis, clinical and structured causality assessments are therefore better approaches than liver histology results obtained through liver biopsy, an invasive procedure with a low complication rate. In rare instances of diagnostic uncertainty regarding alternative causes, liver biopsy should be considered as a final diagnostic approach, provided the patient will profit from this procedure.
Financial SupportThere is no financial support.
Conflict of InterestThere is no conflict of interests.