Dear Editor:
Chronic hepatitis C virus (HCV) infection is the leading reason for liver transplantation in Western countries.1 Unfortunately, reinfection of the graft is universal after transplantation,2 occurring early at reperfusion.3 There is no accepted approach for preventing reinfection, but strategies aimed at blocking viral entry to the hepatocyte seem interesting in this clinical scenario. HCV circulates in human serum associated with various lipoproteins, including HDL, LDL and VLDL, forming lipo-viral particles (LVP),4-6 and among the various entry factors described, several are related to lipoprotein receptors, including LDL receptor7 and scavenger receptor class B type I (SCARB1, also known as SR-BI).8 In 2012 a new entry factor was described: Niemann-Pick C1-like 1 (NPC1L1).9 NPC1L1 is a cholesterol-sensing receptor expressed in enterocytes and in the liver in humans, which can be antagonized by ezetimibe. We were interested in knowing if using ezetimibe for HCV infected patients undergoing a liver transplantation could avoid or delay viral infection of the graft.
For this purpose, a pilot clinical trial was designed to enroll 12 patients with no control arm (given that reinfection is universal). Briefly, patients in the waiting list for a liver transplantation with chronic hepatitis C were invited to participate in this study. The inclusion criteria were:
- •
Chronic hepatitis C defined as detectable HCV RNA for more than 6 months.
- •
Age > 18 years old.
- •
No current HCV antiviral treatment.
- •
No medications for dyslipidemia in the preceding 2 months.
- •
Listed in the national waiting list for liver transplant with an estimated time to transplantation of 3 months or less, either for complications of cirrhosis or for hepatocellular carcinoma.
- •
No abdominal surgery that could alter biliary or intestinal anatomy.
- •
HCV RNA level > 10,000 IU/mL.
- •
No evidence of sitosterolemia.
- •
Negative pregnancy test in urine (for females).
- •
Signed informed consent document.
After screening, patients were started on ezetimibe 10 mg QD po. The study was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT02768545).
We were able to enroll 2 patients after screening five potential participants. The arrival of new oral direct antiviral agents with high chance of sustained virological response during the study period constituted a major barrier for enrollment, so the investigators decided to stop the trial at this point. We present the results of treatment in these two patients.
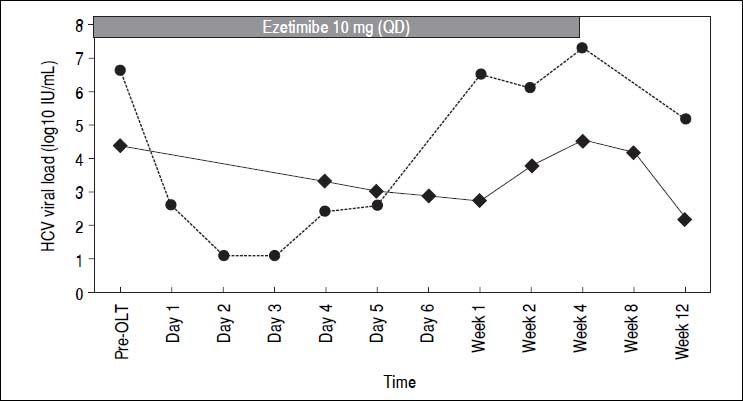
Both patients were female, 65 and 57 years old, both genotype 1b. Liver transplant was indicated because of decompensated liver cirrhosis. Ezetimibe was started 2 and 60 days before the liver transplant, respectively. Ezetimibe was reintroduced the day after transplant in both patients by a nasogastric tube and then orally until week 4. Standard immunosuppression with tacrolimus, prednisone and mycophenolate was administered. Ezetimibe was well tolerated with no adverse events attributed to the medication. HCV viral load is depicted in figure 1 for both patients. It is interesting to note that HCV viral load remained low or even undetectable during the first week after liver transplant, increasing thereafter. At week 4 and afterwards, viral load returned to the pre-transplant values or higher.
The study of Garcia-Retortillo, et al.10 elegantly demonstrated that HCV viral load decreases to a nadir after 8 to 24 h after reperfusion. This first phase probably represents removal of circulating virus by the liver. Subsequently, viral titers increase during the first week, presumably by production of new viral particles in the newly infected virus. In our experience, treatment of these two patients with ezetimibe prior and after liver transplantation shows a tendency to remain in the lower range, being undetectable in day 2 and 3 in one of the patients, and with a steady decrease during the first week in the other patient, using the COBAS TaqMan HCV Test™ v2.0 assay. We were not able to enroll the required number of patients for a more robust analysis of the effect of treatment with ezetimibe after liver transplantation in this pilot trial, but nevertheless our results suggest that blocking HCV entry factors could result in a biological relevant effect. At the same time, this experience shows that blocking only one entry factor at the time of transplantation does not seem to be enough to prevent HCV recurrence in the graft. Maybe a combination of host targeted therapies or the combination of ezetimibe with antiviral agents could have a more profound effect in the outcome of HCV infection after transplantation, as this latter combination has shown synergic effects in vitro.11 The excellent results of treatments with all oral direct antiviral drugs for HCV may fulfill most unmet medical needs, including prevention of graft reinfection and treatment of patients with resistance,12 but the history of eradication of HCV infection is just starting. The lessons learned from other fields such as HIV infection, where in addition to several classes of direct antivirals, at least two entry inhibitors (maraviroc and enfuvirtide) have been developed and proven to be helpful and effective, show that research in additional strategies for HCV control, namely host-targeted agents, cannot be deemed as irrelevant.
GrantsThis study was funded by grant FONDECYT #1130357 from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) to AS.