Data on the efficacy and tolerance of interferon-free treatment in chronic hepatitis C (CHC) in elderly patients are limited in phase II-III trials.
Material and methodsA prospective cohort of adult patients with CHC treated in French general hospitals.
ResultsData from 1,123 patients, distributed into four age groups, were analyzed. Of these, 278 were > 64 years old (fourth quartile) and 133 were > 73 years old (tenth decile). Elderly patients weighed less, were more frequently treatment-experienced women infected with genotype 1b or 2, while they less frequently had genotype 3 or HIV coinfection, but had more frequent comorbidities and drug consumption. Half of the patients had cirrhosis, whatever their ages. The main treatment regimens were sofosbuvir/ledipasvir (37.8%), sofosbuvir/daclatasvir (31.8%), sofosbuvir/simeprevir (16.9%), sofosbuvir/ribavirin (7.8%); ribavirin was given to 24% of patients. The overall sustained virological response (SVR) rate was 91.0 % (95% CI: 89.292.5%) with no difference according to age. Logistic regression of the independent predictors of SVR were albumin, hepatocellular carcinoma and treatment regimen, but not age. The rate of severe adverse events (66 in 59/1062 [5.6%] patients) tended to be greater in patients older than 64 years of age (21/261,8.1%), but the only independent predictors of SAE by logistic regression were cirrhosis and baseline hemoglobin. Patient-reported overall tolerance was excellent in all age groups, and patient-reported fatigue decreased during and after treatment, independent of age.
ConclusionsThe high efficacy and tolerance of interferon-free regimens is confirmed in elderly patients in real-life conditions.
In patients with chronic hepatitis C (CHC), numerous studies have shown that advancing age is generally associated with a poorer sustained virological response (SVR) to interferon-ribavirin treatment, as well as an increase in the discontinuation rate and dose reductions due to adverse events.1,2 Advanced age is also associated with a longer duration of infection, more severe disease, and reduced access to interferon-based regimens.3 Furthermore, the results of real-life studies of interferon-containing treatments are often worse than phase II-III studies, partly because treated patients are older and have more severe liver disease.4,5
Major progress has been made in the treatment of CHC with new direct acting antiviral (DAAs) and, in large trials, age does not seem to modify SVR rates. However, in these phase II and III trials, patients older than 70-75 and those with severe comorbidities were usually excluded. In a post-hoc analysis of four phase III studies in 2,293 patients treated with sofosbuvir (SOF)-ledipasvir (LDV), only 12% were older than 65 and only 1% of these were older than 75.6 Since new DAAs have only been available since 2015, real-life results are very recent.7-19 In France, a large number of elderly patients will probably need to be treated because nearly 40% of undiagnosed patients with hepatitis C belong to the 70-80 year-old age group (around 25,000 people) and could be recruited with optimized screening strategies.20 If the efficacy and tolerance of DAA’s is confirmed in elderly people, then both the age range of treatment and screening should be kept as broad as in recent recommendations.21-23
The aim of this paper was to report the results of CHC treatment with DAAs in a large cohort of patients in real-life conditions in French general hospitals and to analyze the efficacy and tolerance of treatment according to age.
Material and MethodsAPROVVIE is a multicenter, observational study performed by the Association Nationale des Gastroenterologues des Hopitaux generaux (ANGH) in French general hospitals. This study included consecutive adult patients (> 18 years old) treated for chronic hepatitis C (CHC) to evaluate the efficacy of and tolerance to treatment in real-life conditions. This study began on October 10, 2012. After July 4, 2014, investigators could include patients treated with new DAAs, first for early access programs, and then in daily practice.
Inclusion and exclusion criteriaEligible patients were treatment-naive or -experienced adults (at least 18 years-old) with CHC and detectable HCV-RNA treated with an interferon-free regimen. There were no exclusion criteria except patient refusal.
The protocol was performed in accordance with the Declaration of Helsinki and French law for biomedical research, and authorized by the Commission Nationale de l’Informatique et des Libertes (Decision DR-2012-298). Written informed consent was obtained from all patients before beginning the study.
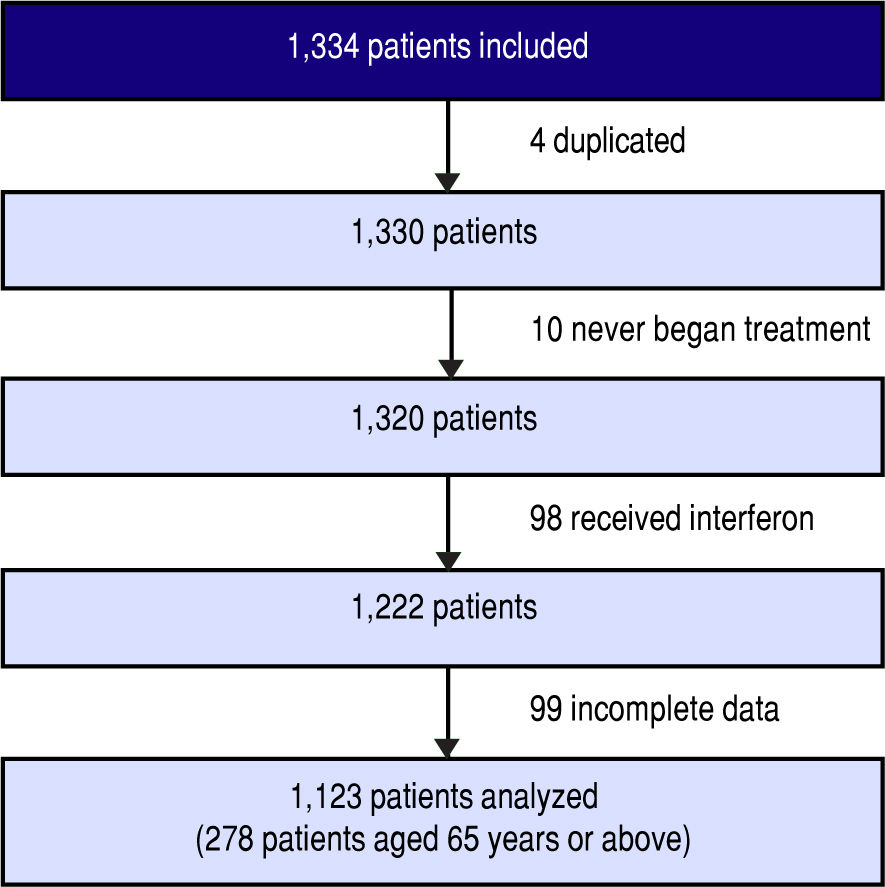
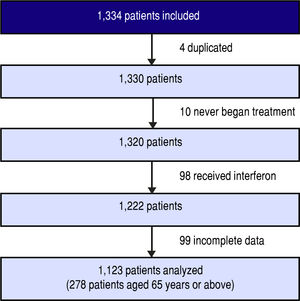
Between March 1, 2014 and January 1, 2016, 1,334 patients were included in the cohort. Four of these were duplicates, 10 never began treatment, 98 received interferon, and 99 had incomplete data due to incomplete records or because their physician left the study.
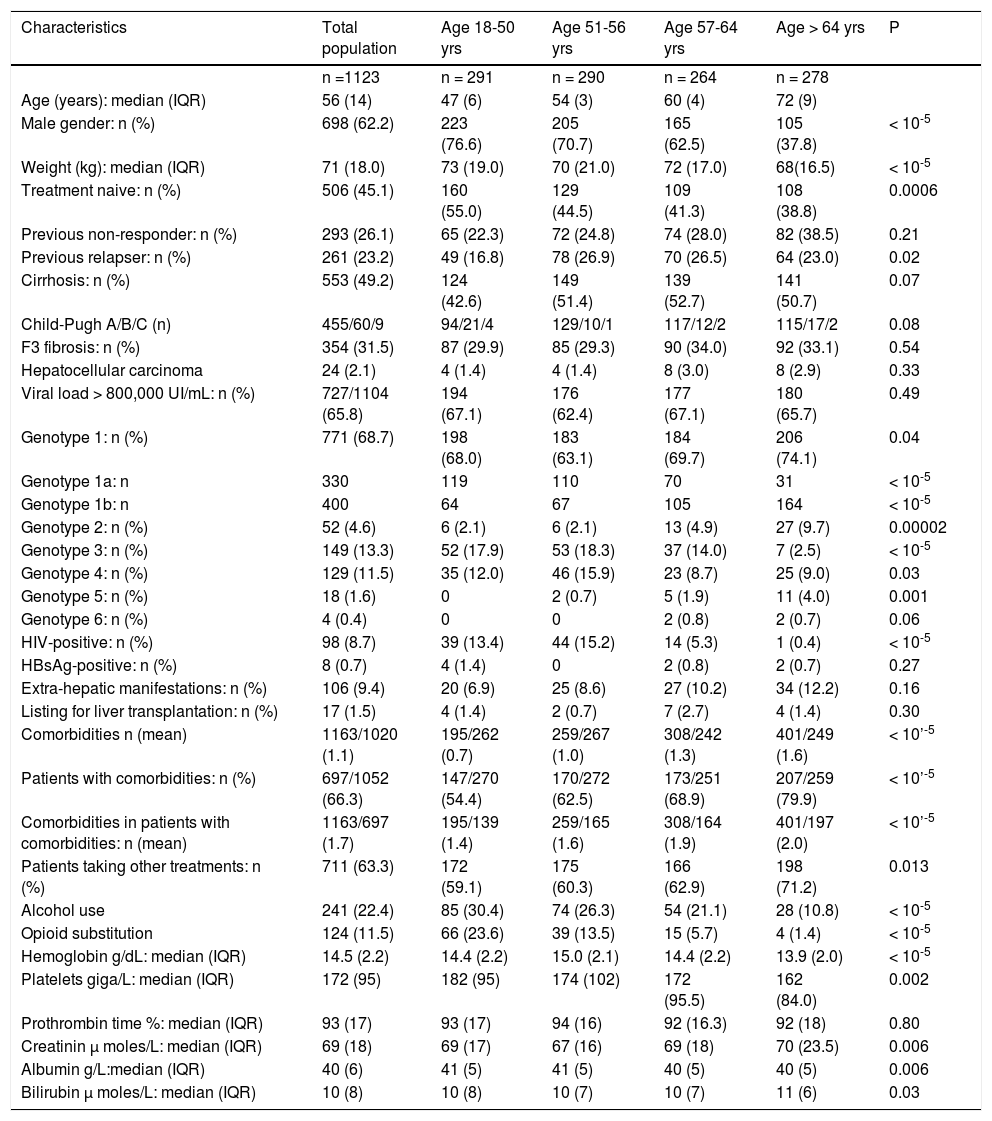
Finally, 1,123 patients were evaluated (Figure 1). The main patient characteristics are reported in table I.
Main characteristics of 1,123 patients with chronic hepatitis C according to age.
| Characteristics | Total population | Age 18-50 yrs | Age 51-56 yrs | Age 57-64 yrs | Age > 64 yrs | P |
|---|---|---|---|---|---|---|
| n =1123 | n = 291 | n = 290 | n = 264 | n = 278 | ||
| Age (years): median (IQR) | 56 (14) | 47 (6) | 54 (3) | 60 (4) | 72 (9) | |
| Male gender: n (%) | 698 (62.2) | 223 (76.6) | 205 (70.7) | 165 (62.5) | 105 (37.8) | < 10-5 |
| Weight (kg): median (IQR) | 71 (18.0) | 73 (19.0) | 70 (21.0) | 72 (17.0) | 68(16.5) | < 10-5 |
| Treatment naive: n (%) | 506 (45.1) | 160 (55.0) | 129 (44.5) | 109 (41.3) | 108 (38.8) | 0.0006 |
| Previous non-responder: n (%) | 293 (26.1) | 65 (22.3) | 72 (24.8) | 74 (28.0) | 82 (38.5) | 0.21 |
| Previous relapser: n (%) | 261 (23.2) | 49 (16.8) | 78 (26.9) | 70 (26.5) | 64 (23.0) | 0.02 |
| Cirrhosis: n (%) | 553 (49.2) | 124 (42.6) | 149 (51.4) | 139 (52.7) | 141 (50.7) | 0.07 |
| Child-Pugh A/B/C (n) | 455/60/9 | 94/21/4 | 129/10/1 | 117/12/2 | 115/17/2 | 0.08 |
| F3 fibrosis: n (%) | 354 (31.5) | 87 (29.9) | 85 (29.3) | 90 (34.0) | 92 (33.1) | 0.54 |
| Hepatocellular carcinoma | 24 (2.1) | 4 (1.4) | 4 (1.4) | 8 (3.0) | 8 (2.9) | 0.33 |
| Viral load > 800,000 UI/mL: n (%) | 727/1104 (65.8) | 194 (67.1) | 176 (62.4) | 177 (67.1) | 180 (65.7) | 0.49 |
| Genotype 1: n (%) | 771 (68.7) | 198 (68.0) | 183 (63.1) | 184 (69.7) | 206 (74.1) | 0.04 |
| Genotype 1a: n | 330 | 119 | 110 | 70 | 31 | < 10-5 |
| Genotype 1b: n | 400 | 64 | 67 | 105 | 164 | < 10-5 |
| Genotype 2: n (%) | 52 (4.6) | 6 (2.1) | 6 (2.1) | 13 (4.9) | 27 (9.7) | 0.00002 |
| Genotype 3: n (%) | 149 (13.3) | 52 (17.9) | 53 (18.3) | 37 (14.0) | 7 (2.5) | < 10-5 |
| Genotype 4: n (%) | 129 (11.5) | 35 (12.0) | 46 (15.9) | 23 (8.7) | 25 (9.0) | 0.03 |
| Genotype 5: n (%) | 18 (1.6) | 0 | 2 (0.7) | 5 (1.9) | 11 (4.0) | 0.001 |
| Genotype 6: n (%) | 4 (0.4) | 0 | 0 | 2 (0.8) | 2 (0.7) | 0.06 |
| HIV-positive: n (%) | 98 (8.7) | 39 (13.4) | 44 (15.2) | 14 (5.3) | 1 (0.4) | < 10-5 |
| HBsAg-positive: n (%) | 8 (0.7) | 4 (1.4) | 0 | 2 (0.8) | 2 (0.7) | 0.27 |
| Extra-hepatic manifestations: n (%) | 106 (9.4) | 20 (6.9) | 25 (8.6) | 27 (10.2) | 34 (12.2) | 0.16 |
| Listing for liver transplantation: n (%) | 17 (1.5) | 4 (1.4) | 2 (0.7) | 7 (2.7) | 4 (1.4) | 0.30 |
| Comorbidities n (mean) | 1163/1020 (1.1) | 195/262 (0.7) | 259/267 (1.0) | 308/242 (1.3) | 401/249 (1.6) | < 10’-5 |
| Patients with comorbidities: n (%) | 697/1052 (66.3) | 147/270 (54.4) | 170/272 (62.5) | 173/251 (68.9) | 207/259 (79.9) | < 10’-5 |
| Comorbidities in patients with comorbidities: n (mean) | 1163/697 (1.7) | 195/139 (1.4) | 259/165 (1.6) | 308/164 (1.9) | 401/197 (2.0) | < 10’-5 |
| Patients taking other treatments: n (%) | 711 (63.3) | 172 (59.1) | 175 (60.3) | 166 (62.9) | 198 (71.2) | 0.013 |
| Alcohol use | 241 (22.4) | 85 (30.4) | 74 (26.3) | 54 (21.1) | 28 (10.8) | < 10-5 |
| Opioid substitution | 124 (11.5) | 66 (23.6) | 39 (13.5) | 15 (5.7) | 4 (1.4) | < 10-5 |
| Hemoglobin g/dL: median (IQR) | 14.5 (2.2) | 14.4 (2.2) | 15.0 (2.1) | 14.4 (2.2) | 13.9 (2.0) | < 10-5 |
| Platelets giga/L: median (IQR) | 172 (95) | 182 (95) | 174 (102) | 172 (95.5) | 162 (84.0) | 0.002 |
| Prothrombin time %: median (IQR) | 93 (17) | 93 (17) | 94 (16) | 92 (16.3) | 92 (18) | 0.80 |
| Creatinin μ moles/L: median (IQR) | 69 (18) | 69 (17) | 67 (16) | 69 (18) | 70 (23.5) | 0.006 |
| Albumin g/L:median (IQR) | 40 (6) | 41 (5) | 41 (5) | 40 (5) | 40 (5) | 0.006 |
| Bilirubin μ moles/L: median (IQR) | 10 (8) | 10 (8) | 10 (7) | 10 (7) | 11 (6) | 0.03 |
The main patient characteristics, their treatment and follow-up results were recorded by investigators on an electronic CRF.
The diagnosis of cirrhosis was made by liver biopsy or non-invasive tests including the Fibrotest®, Fibroscan® or Fibrometer® according to the investigator’s preference and French recommendations.24
Patients’ concomitant baseline comorbidities and medications were recorded.
Standard laboratory tests (including hematological and routine biochemical tests) were performed locally.
Blood HCV-RNA was determined by COBAS AmpliPrep®/COBAS TaqMan® (Roche Molecular Systems, Pleasanton, California, USA), or Abbott Real Time HCV Assay (Abbott Molecular, Des Moines, Illinois, USA), with a lower limit of detection of 15 IU/mL and 12 IU/mL, respectively. A SVR was defined as undetectable HCV RNA at least 12 weeks after the end of treatment. Virological relapse was defined as detectable HCV RNA following undetectable HCV RNA at the end of treatment. A virological breakthrough was defined as an increase from the nadir of HCV RNA of at least one log IU/mL or recurrent HCV RNA.
Tolerance adverse effects and fatigueOnly adverse effects > grade 2, according to Common Terminology Criteria for Adverse Events, version 4.1, were recorded.25 Overall tolerance was assessed by the visual analog scale for each patient at the beginning and the end of treatment. Fatigue was also assessed by the visual analog scale for each patient at the beginning and end of treatment and 3-6 months after the end of treatment.
TreatmentDrugs were prescribed according to the investigator’s preference. Investigators were advised to use treatment schedules proposed by the Association Frangaise pour l’ Etude du Foie, which are regularly updated on its website.23
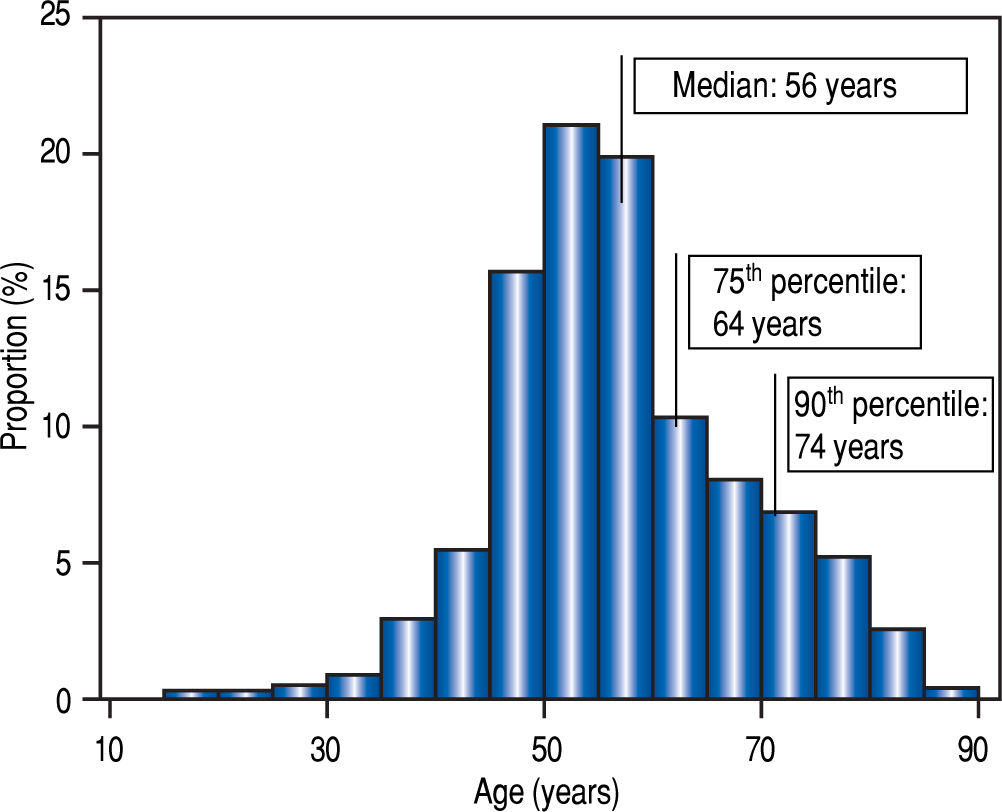
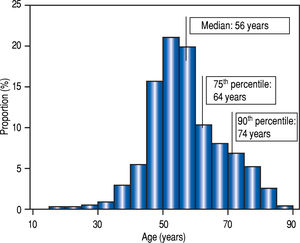
Statistical analysisTo ensure the intrinsic validity of our age categories, the quartiles and deciles of our population were defined. Elderly patients were older than 64 years of age and extremely-old were older than 74 (Figure 2). By chance these thresholds were identical to those used in traditional demographic studies.
Quantitative data were expressed as medians with IQR or means ±SD, according to their distribution, and qualitative data were expressed as numbers and percentages.
Continuous variables were compared using the t-test or Mann-Whitney U test for paired variables when indicated. The Fisher’s exact test or the %2 test was used to analyze categorical variables, and the Armitage test to test trends. Linear regression analysis was used to study the relationship between quantitative variables. To predict the achievement of a SVR, all variables reaching P < 0.10 in univariate analysis were entered into multiple logistic regression analysis. Calculations were made using NCSS 9 statistical software (Kaysville, Utah, USA, www.ncss.com).
Investigators consecutively included all patients treated during the study period. STROBE statements were respected.26
ResultsDescription of the populationAge distribution is shown in figure 2. At the beginning of treatment, the median age was 56 years old (IQR: 14, range18-89). The population was divided into four groups according to age (Table 1, Figure 2).
With advancing age, the sex ratio, the proportion of treatment-naive patients, of patients with genotypes 1a and 3 and of HIV-positive patients decreased while the prevalence of cirrhosis, fibrosis F3, and high viral load were similar (Table 1). Hemoglobin, platelets and albumin tended to be lower with increasing age, but prothrombin time was similar, and blood creatinine and bilirubin higher (Table 1). In the 553 patients with cirrhosis (approximately half of the total population), very few (69, 12.4%) of the patients were decompensated and the distribution of Child-Pugh stages was similar among the classes of age.
The proportion of patients with comorbidities and the number of comorbid conditions increased with age (Table 2). Medication use increased from 59% in patients younger than 51 years old to 71% after age 64 (P = 0.001).
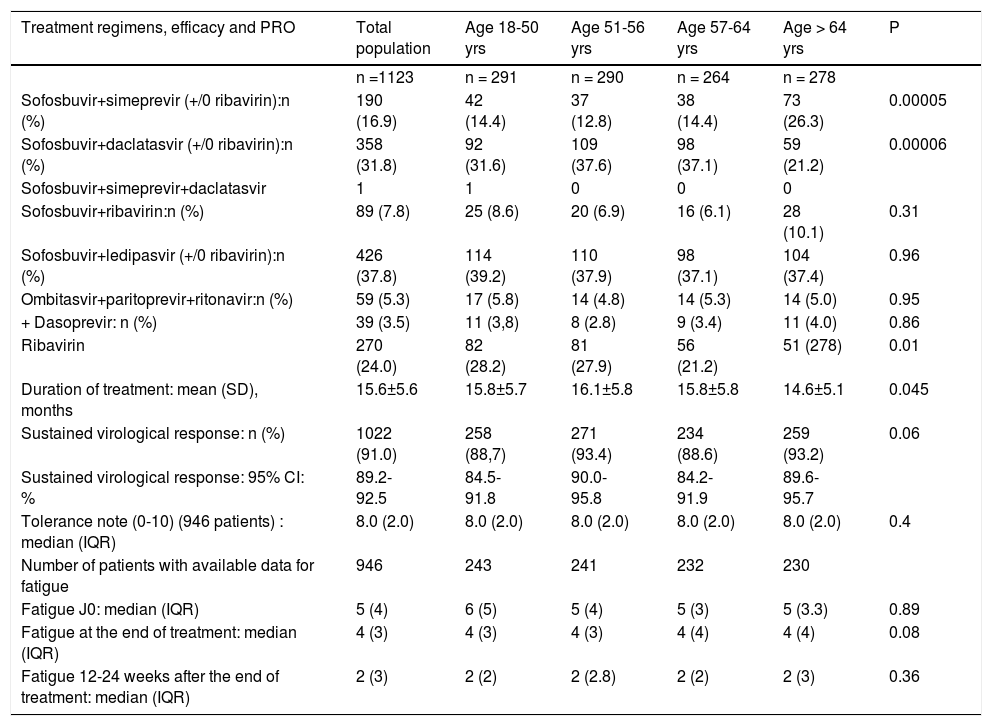
DAAs regimens, SVR rates, patients-reported fatigue and tolerance according to age.
| Treatment regimens, efficacy and PRO | Total population | Age 18-50 yrs | Age 51-56 yrs | Age 57-64 yrs | Age > 64 yrs | P |
|---|---|---|---|---|---|---|
| n =1123 | n = 291 | n = 290 | n = 264 | n = 278 | ||
| Sofosbuvir+simeprevir (+/0 ribavirin):n (%) | 190 (16.9) | 42 (14.4) | 37 (12.8) | 38 (14.4) | 73 (26.3) | 0.00005 |
| Sofosbuvir+daclatasvir (+/0 ribavirin):n (%) | 358 (31.8) | 92 (31.6) | 109 (37.6) | 98 (37.1) | 59 (21.2) | 0.00006 |
| Sofosbuvir+simeprevir+daclatasvir | 1 | 1 | 0 | 0 | 0 | |
| Sofosbuvir+ribavirin:n (%) | 89 (7.8) | 25 (8.6) | 20 (6.9) | 16 (6.1) | 28 (10.1) | 0.31 |
| Sofosbuvir+ledipasvir (+/0 ribavirin):n (%) | 426 (37.8) | 114 (39.2) | 110 (37.9) | 98 (37.1) | 104 (37.4) | 0.96 |
| Ombitasvir+paritoprevir+ritonavir:n (%) | 59 (5.3) | 17 (5.8) | 14 (4.8) | 14 (5.3) | 14 (5.0) | 0.95 |
| + Dasoprevir: n (%) | 39 (3.5) | 11 (3,8) | 8 (2.8) | 9 (3.4) | 11 (4.0) | 0.86 |
| Ribavirin | 270 (24.0) | 82 (28.2) | 81 (27.9) | 56 (21.2) | 51 (278) | 0.01 |
| Duration of treatment: mean (SD), months | 15.6±5.6 | 15.8±5.7 | 16.1±5.8 | 15.8±5.8 | 14.6±5.1 | 0.045 |
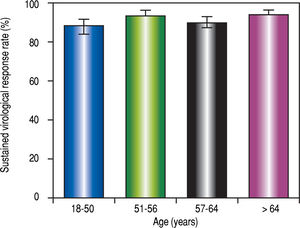
| Sustained virological response: n (%) | 1022 (91.0) | 258 (88,7) | 271 (93.4) | 234 (88.6) | 259 (93.2) | 0.06 |
| Sustained virological response: 95% CI: % | 89.2-92.5 | 84.5-91.8 | 90.0-95.8 | 84.2-91.9 | 89.6-95.7 | |
| Tolerance note (0-10) (946 patients) : median (IQR) | 8.0 (2.0) | 8.0 (2.0) | 8.0 (2.0) | 8.0 (2.0) | 8.0 (2.0) | 0.4 |
| Number of patients with available data for fatigue | 946 | 243 | 241 | 232 | 230 | |
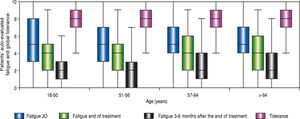
| Fatigue J0: median (IQR) | 5 (4) | 6 (5) | 5 (4) | 5 (3) | 5 (3.3) | 0.89 |
| Fatigue at the end of treatment: median (IQR) | 4 (3) | 4 (3) | 4 (3) | 4 (4) | 4 (4) | 0.08 |
| Fatigue 12-24 weeks after the end of treatment: median (IQR) | 2 (3) | 2 (2) | 2 (2.8) | 2 (2) | 2 (3) | 0.36 |
Alcohol consumption: 241 (22.4%) patients consumed alcohol at the onset of treatment. The proportion of patients abstinent increased regularly with age (from 69% to 89% in patients younger than 51 and those older than 64 years of age, respectively, P < 10-5). The proportion of excess alcohol consumption (> 20 g/d in women, > 30 g/d in men) decreased with age (P = 0.006).
Persistent hard drug abuse was very rare (8 patients, 6 younger than 51 years of age); opioid substitution was infrequent in patients older than 65 (1.4% vs. 14% in patients younger than 65).
New DAAs combinations (Table 2) varied according to genotype and the study period. The less frequent use of sofosbuvir-daclatasvir (DCV) in the oldest patients was due to the low frequency of genotype 3-infected patients compensated by the increased use of sofosbuvir-simeprevir (SIM), which was the most frequently used combination in genotype 1 patients when new DAAs were first used.
Ribavirin (RIB) was less often administered to elderly compared to younger patients (Table 2). The mean duration of treatment was shorter in patients older than 65.
Sustained virological response (SVR)The SVR rate 12-24 weeks after the end of treatment (SVR12-24) was 91.0% (95% CI:89.2-92.5 %). Failures were due to a viral breakthrough (n = 1), relapse after the end of treatment (n = 24) or a severe adverse effect (SAE) (n = 8). One patient was lost to follow-up.
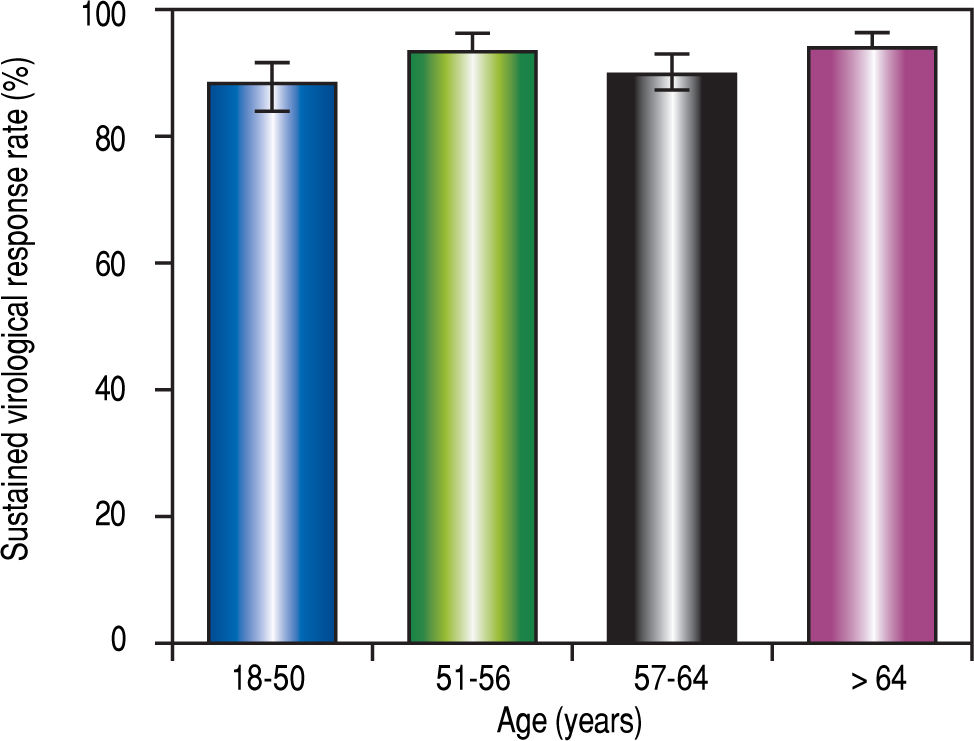
The median age was similar between patients with and without SVR12-24 (57.6 [IQR 15] vs. 57.1 years old [IQR:14), respectively, P = 0.64). However, when the population was distributed into four groups according to age, the SVR12-24 rate was heterogeneous (Table 2 and Figure 3). The %2 test was at the limit of significance. A subgroup analysis showed that the first and third age groups had a lower SVR12-24 rate than the second and fourth age groups (P = 0.04).
Univariate analysis showed that the factors significantly associated with SVR12-24 were cirrhosis (88.3% vs. 93.6%, P = 0.02), the presence of hepatocellular carcinoma (62.5% vs. 91.7%, (P < 10-5), treatment combination (P < 10-5), body weight (P = 0.04), hemoglobin (P = 0 .01), platelets (P = 0.05), prothrombin time (P = 0.005), and albumin (P = 0.00005).
Logistic regression analysis showed that the independent predictive factors of SVR12-24 were albumin(OR:1.05; CI 95%: 1.00-1.10, P = 0.03), hepatocellular carcinoma (OR:0.195 ; CI95%: 0.07-0.52), P = 0.001); body weight (OR:0.97; 95%CI: 0.96-0.99, P = 0.0006); SOF/ SIM (OR:2.84, CI95%: 1.17-6.89, P = 0.02), SOF/ DCV(OR 7.67; CI95%: 3.51-16.78, P < 10-5), SOF/LDV (OR 4.69; CI95%: 2.18-11.29, P = 10-4) (the reference for treatment was SOF/RIBA). When age was retained as a continuous variable this did not change the results. Age set as a categorical variable (4 classes) lead to a significantly independent predictor of success with age greater than 64 years (OR:2.02; IC95%1.02-4.02, P = 0.045), the reference age class being 18-51 years-old one, without substantial variation in the significance of other associated factors.
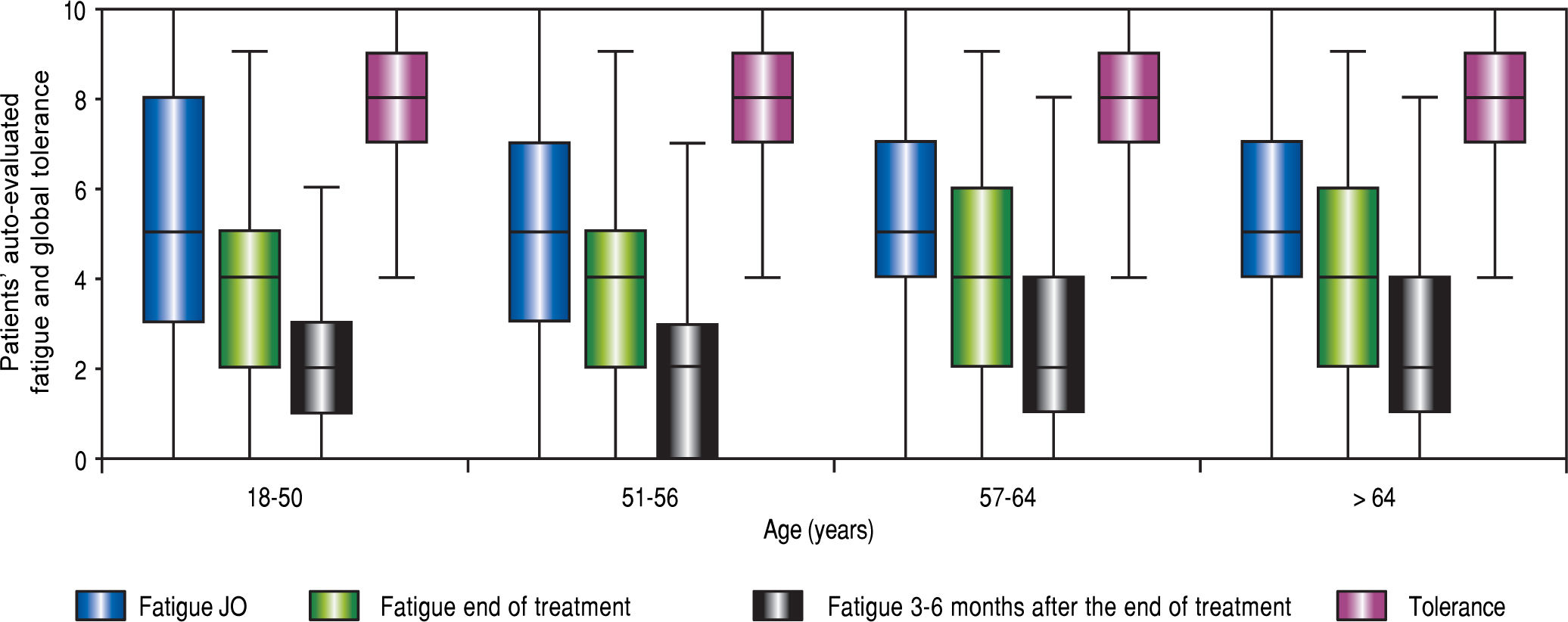
Tolerance(Table 2, Table 3 and Figure 4) Self-assessed global tolerance was excellent (median 8/ 10, IQR 2.0) and identical in all ages. Self-assessed fatigue was similar at the beginning of treatment and decreased during and after the end of treatment in all classes of age.
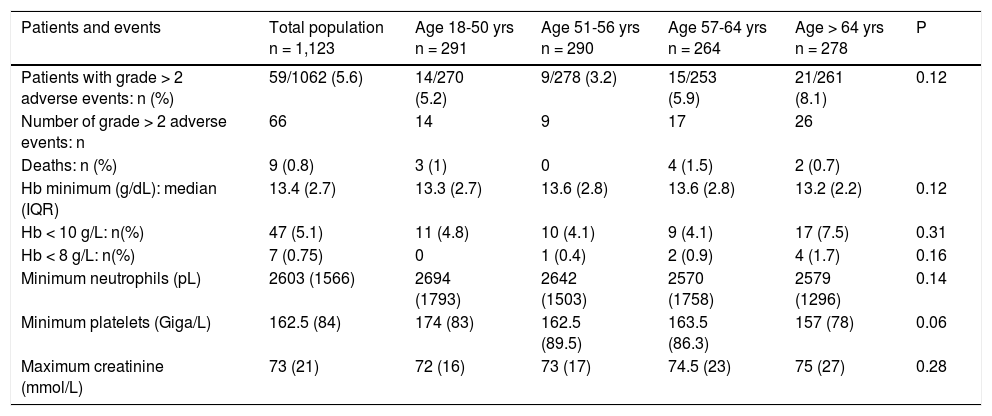
Adverse events according to age.
| Patients and events | Total population n = 1,123 | Age 18-50 yrs n = 291 | Age 51-56 yrs n = 290 | Age 57-64 yrs n = 264 | Age > 64 yrs n = 278 | P |
|---|---|---|---|---|---|---|
| Patients with grade > 2 adverse events: n (%) | 59/1062 (5.6) | 14/270 (5.2) | 9/278 (3.2) | 15/253 (5.9) | 21/261 (8.1) | 0.12 |
| Number of grade > 2 adverse events: n | 66 | 14 | 9 | 17 | 26 | |
| Deaths: n (%) | 9 (0.8) | 3 (1) | 0 | 4 (1.5) | 2 (0.7) | |
| Hb minimum (g/dL): median (IQR) | 13.4 (2.7) | 13.3 (2.7) | 13.6 (2.8) | 13.6 (2.8) | 13.2 (2.2) | 0.12 |
| Hb < 10 g/L: n(%) | 47 (5.1) | 11 (4.8) | 10 (4.1) | 9 (4.1) | 17 (7.5) | 0.31 |
| Hb < 8 g/L: n(%) | 7 (0.75) | 0 | 1 (0.4) | 2 (0.9) | 4 (1.7) | 0.16 |
| Minimum neutrophils (pL) | 2603 (1566) | 2694 (1793) | 2642 (1503) | 2570 (1758) | 2579 (1296) | 0.14 |
| Minimum platelets (Giga/L) | 162.5 (84) | 174 (83) | 162.5 (89.5) | 163.5 (86.3) | 157 (78) | 0.06 |
| Maximum creatinine (mmol/L) | 73 (21) | 72 (16) | 73 (17) | 74.5 (23) | 75 (27) | 0.28 |
Sixty-six adverse events > grade 2 were observed in 59 (5.6%) patients, with no significant variation for age (Table 3). There were 9 deaths, all in patients with cirrhosis (3 from hepatocellular carcinoma, 2 from decompensation and 1 from metastatic undifferentiated carcinoma, cardiac failure, renal failure, and septic shock, respectively). There was no significant difference among age groups for minimum hemoglobin, neutrophils or maximum creatinine during treatment. However, severe anemia (Hb < 10 g/dL) was observed in 7.5% of patients aged of 65 years or more vs. 4.3% in patients < 65 years (P = 0.06) The median decrease in hemoglobin was greater in patients receiving ribavirin (2.2 g/dL, IQR 2.5) than those not receiving this drug (0.6 g/dL, IQR 1.2)(P < 10-5). Logistic regression analysis identified ribavirin (OR:8.90, 95% CI:4.12-19.34, P < 10-5), baseline Hemoglobin (OR:0.35; 95%CI:0.27-0.45, P < 10-5), age > 65 years being borderline significant (OR 2.63; 95%CI: 0.96-7.23, P = 0.06) Minimum platelets tended to be lower in the oldest age group.
Univariate analysis showed that the factors significantly associated with the occurrence of adverse events greater than grade 2 were cirrhosis (9.2% vs. 1.9%, P = 0.00002), hepatocellular carcinoma (25.0% vs. 5 %, P = 0.00002), the presence of comorbidities (6.8% vs. 3.5%, P = 0.03), the use of ribavirin (8.5% vs. 4.2%, P = 0.007), the type of combination therapy (P = 0.06), baseline hemoglobin (13.3 vs. 14.4 g/dL, P = 10-5), neutrophils (P = 0.05), platelets (P = 0.0002), albumin (P = 0.0001) and bilirubin (P = 0.0007).
The independent predictive factors for adverse events greater than grade 2 were the presence of cirrhosis (OR 3.23; 95% CI : 1.41-7.38, P = 0.005) and baseline hemoglobin (0R 0.81; 95% CI: 0.69-0.97, P = 0.01). Using age as either a continuous variable or as a dichotomous categorical variable (patients aged of 65 years or more vs. patients aged less than 65 years) did not change the results of logistic regression).
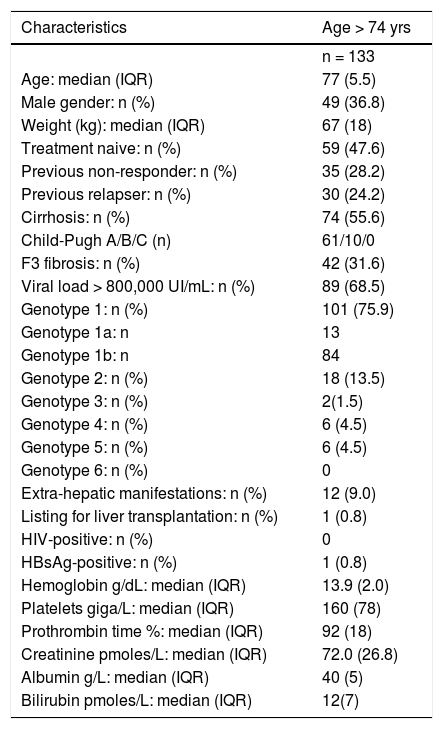
Subgroup of patients over 73 years-old(Tables 4 and 5)There were 133 patients over 73 years-old (oldest age group). They were more often treatment-naive with geno-type 1 infection, more frequently had cirrhosis, and lower baseline albumin and bilirubin. Their overall SVR12-24 rate (91.7 %), and the occurrence of adverse events were similar to the 65-73 year-old group.
Main characteristics of patients aged > 74 years.
| Characteristics | Age > 74 yrs |
|---|---|
| n = 133 | |
| Age: median (IQR) | 77 (5.5) |
| Male gender: n (%) | 49 (36.8) |
| Weight (kg): median (IQR) | 67 (18) |
| Treatment naive: n (%) | 59 (47.6) |
| Previous non-responder: n (%) | 35 (28.2) |
| Previous relapser: n (%) | 30 (24.2) |
| Cirrhosis: n (%) | 74 (55.6) |
| Child-Pugh A/B/C (n) | 61/10/0 |
| F3 fibrosis: n (%) | 42 (31.6) |
| Viral load > 800,000 UI/mL: n (%) | 89 (68.5) |
| Genotype 1: n (%) | 101 (75.9) |
| Genotype 1a: n | 13 |
| Genotype 1b: n | 84 |
| Genotype 2: n (%) | 18 (13.5) |
| Genotype 3: n (%) | 2(1.5) |
| Genotype 4: n (%) | 6 (4.5) |
| Genotype 5: n (%) | 6 (4.5) |
| Genotype 6: n (%) | 0 |
| Extra-hepatic manifestations: n (%) | 12 (9.0) |
| Listing for liver transplantation: n (%) | 1 (0.8) |
| HIV-positive: n (%) | 0 |
| HBsAg-positive: n (%) | 1 (0.8) |
| Hemoglobin g/dL: median (IQR) | 13.9 (2.0) |
| Platelets giga/L: median (IQR) | 160 (78) |
| Prothrombin time %: median (IQR) | 92 (18) |
| Creatinine pmoles/L: median (IQR) | 72.0 (26.8) |
| Albumin g/L: median (IQR) | 40 (5) |
| Bilirubin pmoles/L: median (IQR) | 12(7) |
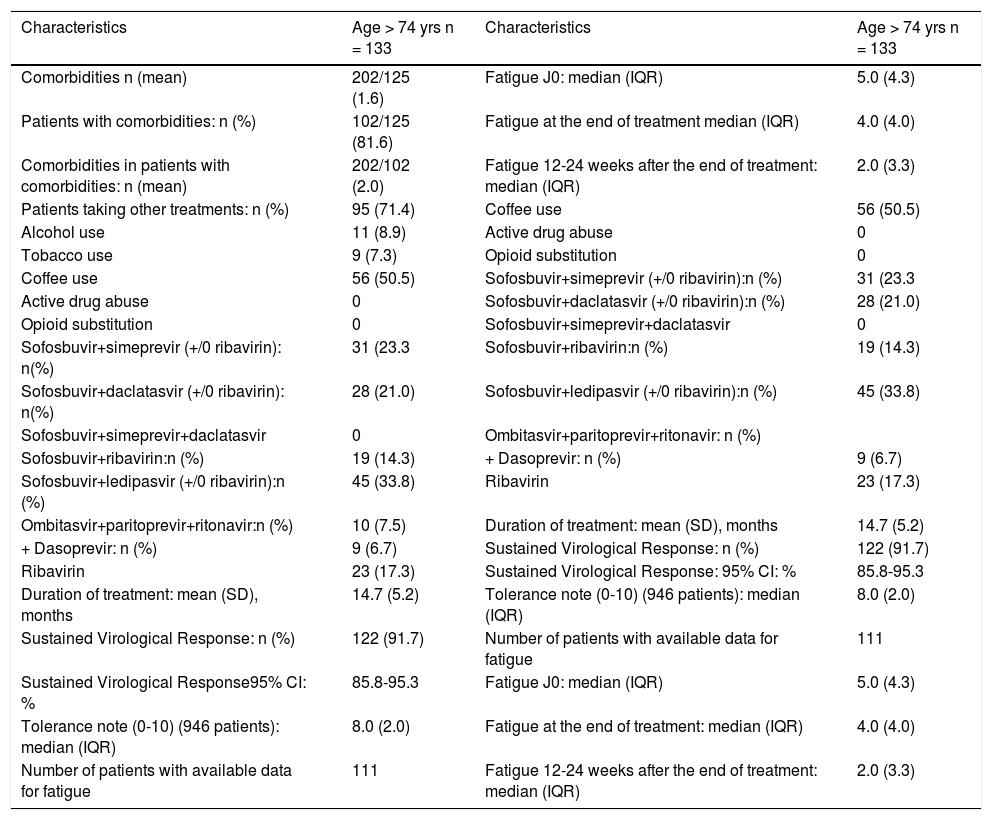
Comorbidities, treatment, DAAs regimens, sustained virological rates, patient-reported fatigue and tolerance in patients > 74 years old.
| Characteristics | Age > 74 yrs n = 133 | Characteristics | Age > 74 yrs n = 133 |
|---|---|---|---|
| Comorbidities n (mean) | 202/125 (1.6) | Fatigue J0: median (IQR) | 5.0 (4.3) |
| Patients with comorbidities: n (%) | 102/125 (81.6) | Fatigue at the end of treatment median (IQR) | 4.0 (4.0) |
| Comorbidities in patients with comorbidities: n (mean) | 202/102 (2.0) | Fatigue 12-24 weeks after the end of treatment: median (IQR) | 2.0 (3.3) |
| Patients taking other treatments: n (%) | 95 (71.4) | Coffee use | 56 (50.5) |
| Alcohol use | 11 (8.9) | Active drug abuse | 0 |
| Tobacco use | 9 (7.3) | Opioid substitution | 0 |
| Coffee use | 56 (50.5) | Sofosbuvir+simeprevir (+/0 ribavirin):n (%) | 31 (23.3 |
| Active drug abuse | 0 | Sofosbuvir+daclatasvir (+/0 ribavirin):n (%) | 28 (21.0) |
| Opioid substitution | 0 | Sofosbuvir+simeprevir+daclatasvir | 0 |
| Sofosbuvir+simeprevir (+/0 ribavirin): n(%) | 31 (23.3 | Sofosbuvir+ribavirin:n (%) | 19 (14.3) |
| Sofosbuvir+daclatasvir (+/0 ribavirin): n(%) | 28 (21.0) | Sofosbuvir+ledipasvir (+/0 ribavirin):n (%) | 45 (33.8) |
| Sofosbuvir+simeprevir+daclatasvir | 0 | Ombitasvir+paritoprevir+ritonavir: n (%) | |
| Sofosbuvir+ribavirin:n (%) | 19 (14.3) | + Dasoprevir: n (%) | 9 (6.7) |
| Sofosbuvir+ledipasvir (+/0 ribavirin):n (%) | 45 (33.8) | Ribavirin | 23 (17.3) |
| Ombitasvir+paritoprevir+ritonavir:n (%) | 10 (7.5) | Duration of treatment: mean (SD), months | 14.7 (5.2) |
| + Dasoprevir: n (%) | 9 (6.7) | Sustained Virological Response: n (%) | 122 (91.7) |
| Ribavirin | 23 (17.3) | Sustained Virological Response: 95% CI: % | 85.8-95.3 |
| Duration of treatment: mean (SD), months | 14.7 (5.2) | Tolerance note (0-10) (946 patients): median (IQR) | 8.0 (2.0) |
| Sustained Virological Response: n (%) | 122 (91.7) | Number of patients with available data for fatigue | 111 |
| Sustained Virological Response95% CI: % | 85.8-95.3 | Fatigue J0: median (IQR) | 5.0 (4.3) |
| Tolerance note (0-10) (946 patients): median (IQR) | 8.0 (2.0) | Fatigue at the end of treatment: median (IQR) | 4.0 (4.0) |
| Number of patients with available data for fatigue | 111 | Fatigue 12-24 weeks after the end of treatment: median (IQR) | 2.0 (3.3) |
The availability of new DAAs represents major progress in the treatment of hepatitis C. The overall SVR rate in this large unselected population of patients treated in general hospitals was 90% independent of age, with a variety of -even suboptimal-drug combinations.
The age groups used in this study were based on the distribution of age in our population. The fourth quartile (> 64 years-old) corresponds well to the definition of elderly patients (> 65 years), and the upper tenth decile to “extremely-old” patients [> 75 years). The distribution of age in our population could have been shifted to the right by the restriction by health authorities of using DAA’s in patients with severe liver lesions.
The effect of age alone is difficult to estimate because chronic hepatitis C is different at different ages, depending on sex-ratios, body weight, genotype, severity of liver lesions and comorbidities. Age could also increase the exposure to and efficacy of SOF due to decreased glomerular filtration rate.
Thirteen studies on the effect of age on the efficacy and tolerance of interferon-free treatments were published in 2016-2017 from Japan, Germany, USA, Italy and Spain based on “real-world” data.7-19 These studies differ in their design (prospective or retrospective), the nature of data (directly from investigators or administrative,9 the number of registered “elderly” ( > 65 years old) patients (from 84 to 4,787), HCV genotype, the proportion of patients with cirrhosis, and the combination of drugs used. However, age did not appear to be a predictive factor for SVR overall or in the various subgroups (genotype, cirrhosis, treatment combinations), in any7-16,18,19 but one17 of these studies. Multivariate analysis was performed in a minority of these studies.9,16,18 Independent predictors of SVR, as in our study, were related to the severity of cirrhosis: albuminemia18 or MELD score.16
Moreover, tolerance did not decrease with age and the prevalence of adverse effects above grade 2, which occurred almost always in patients with cirrhosis, was not different among the classes of age, except in the detailed study of Lens et al. wherein SAE markedly increased when age was > 75 years in univariate analysis (8.8% among those aged 65-74 years compared with 13% and 14% among those aged 75-79 and > 80 years, respectively), and death was independently predicted by albuminemia < 35 g/L and age > 75 years (in multivariate analysis).18
Severe anemia was more frequent in elderly patients when ribavirin was used, and dosage reduction or discontinuation was indicated, usually with no loss of efficacy.7,12,14
This combination of virological efficacy and excellent tolerance probably explains why the age differences observed with interferon-based treatments were strikingly reduced or abolished as well as the differences between the phase III and “real-world” study results.1,2,27
As expected, the proportion of patients with comorbidities and taking concomitant medications increased with age. The number of medications taken probably also increased as in other studies8,11,18 making the risk of clinically significant drug-drug interactions more frequent in older patients.8,18 In observational studies, the treated patients are obviously selected, even if there is no imposed limit on age. Unfortunately, we have no data on the number and characteristics of elderly patients who were not treated in the participating centers during the study period.
In a post-hoc analysis of 8 multinational multicenter phase II and III trials, patient-reported outcomes improved significantly and similarly in all patients during and after treatment with SOF and/or RIBA or LDV, even in patients older than 65.28 In our study, self-assessed fatigue decreased in a similar manner during and after treatment whatever the age.
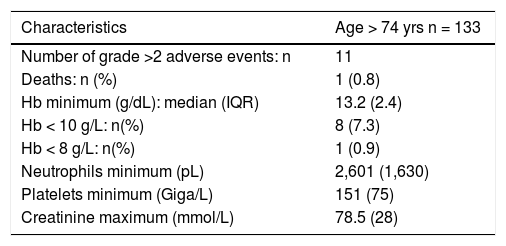
Adverse events in patients > 74 years old
| Characteristics | Age > 74 yrs n = 133 |
|---|---|
| Number of grade >2 adverse events: n | 11 |
| Deaths: n (%) | 1 (0.8) |
| Hb minimum (g/dL): median (IQR) | 13.2 (2.4) |
| Hb < 10 g/L: n(%) | 8 (7.3) |
| Hb < 8 g/L: n(%) | 1 (0.9) |
| Neutrophils minimum (pL) | 2,601 (1,630) |
| Platelets minimum (Giga/L) | 151 (75) |
| Creatinine maximum (mmol/L) | 78.5 (28) |
Although the cost-effectiveness ratio of DAAs increased with age, cost-effectiveness was good in elderly patients with advanced fibrosis in an Italian modelling study. Frailty was a major determinant of cost-effectiveness29 and it appears to be essential in the clinical indications for treatment.
Our study contains several strengths and inherent limitations. The main limitations of this study are its observational design and electronic data collection which may result in a potential physician prescribing bias, incomplete records, local practice discrepancies, data entry errors, missing data and the absence of information on untreated patients during the same period. Furthermore, we did not routinely assess frailty status in our elderly patients. Finally, we do not have any data on the proportion of elderly patients who were not treated during the same period.
The main strength of this study is that it includes a large, unselected population with no specific exclusion criteria, studied under real-life conditions, outside of University hospitals, including all genotypes and various drug combinations to provide objective results on efficacy and tolerance as well as patient-reported outcomes on the progression of fatigue and overall tolerance.
ConclusionsIn conclusion this prospective, observational, multicenter study performed in French general hospitals confirms that age does not result in a decrease in the sustained virological response rate or safety with new DAAs in patients with chronic hepatitis C while fatigue decreases in a similar manner as younger patients. This study suggests that age per se should not be considered a contraindication to antiviral treatment, however frailty, comorbidities and associated treatments should be carefully evaluated. The efficacy of treatment in preventing liver-related complications and survival must be confirmed in further studies. Finally, these results must be confirmed in therapeutic regimens with restricted durations or new molecules.
Abbreviations- •
ANGH: Association Nationale des Gastroenterologues des Hopitaux Generaux.
- •
CHC: chronic hepatitis C.
- •
DAA: Direct-acting antiviral.
- •
DCV: daclatasvir.
- •
LDV: ledipasvir.
- •
RIBA: ribavirin.
- •
SAE: evere adverse effect.
- •
SIM: simeprevir.
- •
SOF: sofosbuvir.
- •
SVR: sustained virological response.
We thank Ms Dale Roche-Lebrec who assisted with language review, and Pr Dermot O’Toole for his friendly ultimate review.
Grants and Financial SupportsJanssen and then Gilead funded the APROVVIE observatory, but had no role in analysis, interpretation of data and redaction, neither in the decision to submit this article for publication.
Conflicts of InterestAlexandre Pariente : Intercept, Mayoly-Spindler, Gilead, Abbvie. Jean-Pierre Arpurt: Gilead, Abbvie, Intercept. Andre-Jean Remy: Abbvie, Gilead, MSD, Janssen, BMS. Isabelle Rosa-Hezode: Abbvie, Gilead, BMS, MSD, Janssen. Xavier Causse: AbbVie, Gilead, Intercept, MSD, Roche. Christophe Renou: AbbVie, Gilead, Intercept, MSD. Christophe Pilette: Abbvie Ramuntxo Arotgarena: Gilead, MSD, Abbvie, Intercept.
Georges Barjonet. Janssen, BMS, Abbvie, MSD, Gilead. Frangois Bourhis: Gilead Abbvie. Arnaud Pauwels: Gilead, Abvvie et MSD. Yann Le Bricquir: Gilead, Abvvie et MSD. Stephanie De Montigny-Lenhart: Abbvie, Gilead, MSD, Roche Jean-Frangois Cadranel: BMS, MSD, Gilead. Herve Hagege: Abbvie, Gilead, BMS, MSD, Janssen. Frederic Heluwaert, Gilles Macaigne, Jean Henrion, Matthieu Schnee, Hatem Salloum, Severine Hommel, Hortensia Lison, Vincent Jouannaud, Edmond Geagea, Bertrand Condat, Marie-Pierre Ripault, David Zanditenas, Helene Labadie, Bertrand Tissot, Eric Maringe and Bruno Lesgourgues did not disclose any conflict of interest.