Aim. Anemia is the most common adverse event in patients with chronic hepatitis C virus (HCV) treated with telaprevir (TVR) combined triple therapy. We examined the effects of drug dose adjustment on anemia and a sustained viral response (sVR) during combination therapy.
Material and methods. This study enrolled 62 patients treated with TVR (2,250 mg) for 12 weeks plus pegylated interferon-alpha-2b and ribavirin for 24 weeks. The patients were assigned randomly to the TVR-standard or -reduced groups before treatment. At the occurrence of anemia (hemoglobin < 12 g/dL), the TVR-reduced group received 1500 mg TVR plus the standard dose of ribavirin, whereas the TVR-standard group received the standard TVR dose (2,250 mg) and a reduced dose of ribavirin (200 mg lower than prescribed originally). The safety and SVR at 24 weeks were compared between the TVR-standard (n = 28) and TVR-reduced (n = 25) groups.
Results. No differences in the proportion of patients who became HCV RNA-negative were detected between the TVR-standard and -reduced groups (72 and 72% at week 4, 79 and 84% at the end of treatment, and 76 and 80% at SVR24, respectively). Two groups had comparable numbers of adverse events, which led to the discontinuation of TVR in 14 patients of TVR-standard group and in 14 of TVR-reduced group. A lower incidence of renal impairment was observed in the TVR-reduced group (6%) than the TVR-standard group (11%, not statistically significant).
Conclusions. TVR dose adjustment could prevent anemia progression without weakening the anti-viral effect during triple therapy in HCV-patients.
Chronic hepatitis C (CHC) due to infection with the hepatitis C virus (HCV) affects ~170 million people worldwide and is the most common cause of chronic liver disease.1 Of HCV-infected individuals, 20 to 30% eventually develop liver cirrhosis or hepatocellular carcinoma (HCC). Elimination of HCV is important for preventing HCC, especially in elderly patients with advanced fibrosis or CHC.2,3 A combination of pegylated-interferon (PEG-IFN) and ribavirin (RBV) completely eradicates HCV in up to 40-50% of treatment-naïve patients with high viral loads of HCV genotype 1b.4,5 Elongation of the treatment period or retreatment improves the rate of sustained virological response (SVR) in some patients with CHC, but not all.6,7
Telaprevir (TVR), a first-generation inhibitor of the NS3/4A serine protease, was approved as a directacting antiviral (DAA) against CHC.8,9 Previous studies have demonstrated significantly higher SVR rates in both treatment-naïve and treatment-experienced CHC subjects with genotype 1 who were treated with TVR-combined triple therapy as compared to those treated with PEG-IFN and RBV combination therapy.8–14 In Japan, TVR-based triple therapy was approved in September 2011, and the use of TVR in combination with PEG-IFN and RBV is currently considered an accepted standard of care in genotype 1 HCV-infected patients.15,16 However, severe anemia and other serious adverse events (SAEs) can occur more frequently in patients with CHC undergoing TVR-combined triple therapy.10,11,17 Initially, the recommended pharmacological drug dose of TVR was 2,250 mg, and dose reduction was not adjusted for adverse events.18,19 By contrast, the recommended dose of RBV was adjusted according to the body weight of patients; furthermore, a dose reduction was recommended in cases with anemia. After the approval of TVR combined triple therapy, the efficacy and safety of the TVR dose reduction to 1,500 mg was discussed in Japan. However, sufficient evidence was not available to confirm the efficacy and safety of TVR dose adjustment therapy. The aims of the present study were to evaluate the anti-HCV effect and safety of TVR dose adjustment for anemia in patients with CHC treated with TVR combined triple therapy.
Material and MethodsPatientsThis study enrolled adult patients, aged 20 to 70 years, who were chronically infected with HCV genotype 1 and had serum HCV RNA viral load > 5 log copies/mL at screening. Additional inclusion criteria were platelet count > 90,000 mm3, and hemoglobin > 12 g/dL. Patients were excluded if they had a medical contraindication to PEG-IFN or RBV, a history of drug use or documented cirrhosis, evidence of other significant liver diseases including hepatitis B virus, autoimmune liver diseases, alcohol liver disease and HCC. No patients had evidence of human immunodeficiency virus infection. This study was conducted according to the guidelines of the 1975 Declaration of Helsinki (2004 version), and written informed consent was obtained from all patients prior to treatment. The study protocol was approved by the Ethics Committee in each hospital and by the Ethics Committee of Osaka City University Graduate School of Medicine (No. 2212). The trial was registered at UMIN (No. 000007071).
Study designPatients from three hospitals were evaluated in a randomized two-arm study. The efficacy and safety of drug dose adjustment for patients with anemia were evaluated in patients receiving TVR (Telavic; Mitsubishi Tanabe Pharma, Osaka, Japan) in combination with PEG-IFN a2b (1.5 µg/kg/week Peg-Intron; MSD, Tokyo, Japan) and RBV (Rebetol, MSD) for 12 weeks using the RBV clearance-based dosage20,21 as follows: 400 mg/day for patients with an oral clearance rate (CL/F) < 8 L/h; 600 mg/day for patients with CL/F ranging from 8 to < 15 L/h, 800 mg/day for patients with CL/F ranging from 15 to < 25 L/h, 1,000 mg/day for patients CL/F more than 25 L/h for 12 weeks, followed by PEG-IFN a2b and RBV for 12 weeks. TVR was initiated at a dose of 750 mg every 8 h (2,250 mg/ day). Before initiating treatment, enrolled patients were allocated randomly into two groups. At the occurrence of anemia (hemoglobin < 12 g/dL), the TVR-standard group (group A) received the standard TVR dose (2,250 mg/day) and a reduced dose of ribavirin that was 200 mg lower than that originally prescribed according to the recommended guideline. The dose of TVR was reduced from 2,250 to 1,500 mg/day without reduction of RBV dose in the TVR-reduced group (group B). After the first reduction in the TVR dose, if the hemoglobin decreased to below 10 g/dL, 200 mg of RBV was reduced from the initiation dose according to the recommended guidelines. When other hematologic side effects occurred, the PEG-IFN a2b dose was reduced according to the drug recommendations. During treatment, no patients received erythropoietin or granulocyte-macrophage colony stimulating factor. For patients with grade 1 (one or several sites affected) or grade 2 (diffuse skin eruptions involving up to 50% of the body surface) dermatological adverse events, medical management was performed at the discretion of the physicians at each hospital. If a progressive grade 3 dermatological adverse event developed (rash with the appearance of substantial systemic signs or symptoms or involving more than 50% of the body surface), TVR was discontinued; however, the patients continued to receive PEG-IFNa2b and RBV if possible.
Virologic evaluationsHCV-RNA was determined using the TaqMan HCV assay (COBAS® TaqMan® HCV assay; Roche Molecular Diagnostics, Tokyo, Japan) with a lower limit of quantification of 15 IU/mL and an upper limit of quantification of 6.9 x 107 IU/mL (1.2-7.8 log IU/mL). The HCV genotype was determined using a HCV genotype primer kit (Institute of Immunology Co., Ltd., Tokyo, Japan).
A previous viral response to IFN-based therapy was defined as follows: prior relapse showing undetectable HCV RNA at the end of treatment but detectable HCV RNA within 24 weeks after the end of treatment; and the re-appearance of HCV RNA at any time during treatment after a viral response (breakthrough). Patients with HCV-RNA that remained detectable during treatment were defined as non-responders.
Assessment of treatment efficacyRapid viral response (RVR) was defined as undetectable serum HCV-RNA at week 4. End-of-treatment response (ETR) was defined as undetectable HCV-RNA at the end of therapy. SVR 24 was defined as undetectable HCV-RNA at 24 weeks after completion of treatment. All methods of assessing treatment efficacy were defined according to the guidelines.15 During the follow-up period, clinical, biochemical and quantitative serum HCV-RNA assessments were evaluated at 1-3-month intervals.
SNP genostypingWe examined the genetic polymorphisms in the IL28B and ITPA genes only in patients who consented to genetic analysis.22,23 Whole blood was collected from patients and centrifuged to separate the buffy coat. Genomic DNA was extracted from the buffy coat using a QIAamp® DNA Blood Midi Kit (QIAGEN, Maryland, USA). The genetic polymorphisms in IL28B rs8099917 and rs12979860 and ITPA rs1127354 were genotyped using the TaqMan SNP Genotyping Assay with the 7500 Fast RealTime PCR System (Applied Biosystems, Foster City, CA, USA). All samples were also genotyped by direct sequencing to confirm the genotype. The primers and procedures used were described previously.7
Statistical analysisData analyses were conducted using the JMP software, ver. 9.0 (SAS Institute, Cary, NC, USA). Characteristics between groups were evaluated by Wilcoxon’s two-sample test for numerical variables or Fisher’s exact test for categorical variables. Variables exhibiting values of p < 0.1 on univariate analysis were subjected to stepwise multivariate logistic regression analysis. In the two-tailed test, p < 0.05 was taken to indicate statistical significance.
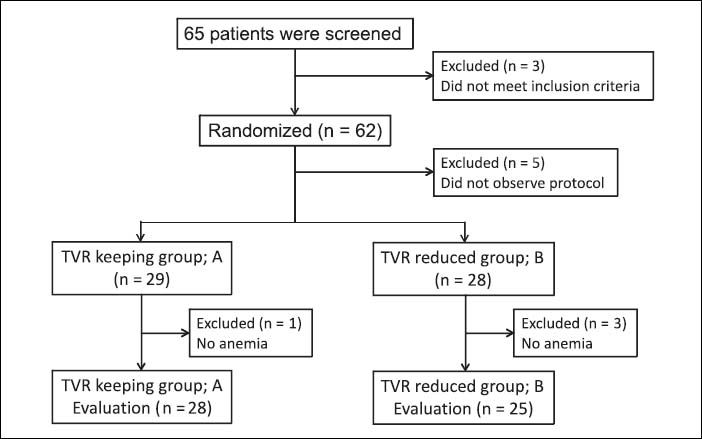
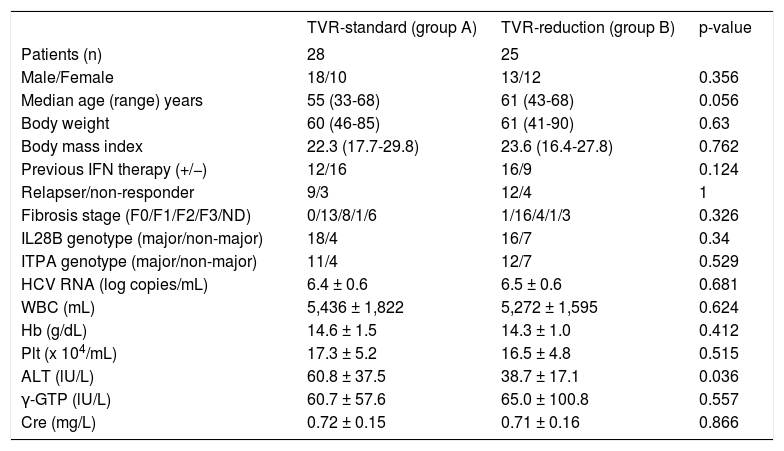
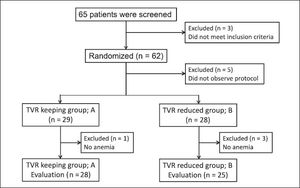
ResultsPatientsSixty-five patients were screened for enrollment in this study: eight patients were excluded because they did not observe the study protocol and 57 patients were randomly allocated to two groups and followed-up until SVR determination. The TVR-standard group (group A) contained 29 patients, and it was necessary to reduce the RBV dose during treatment in 28 of these patients. The TVR-reduced group (group B) contained 28 patients, and it was necessary to reduce the TVR dose in 25 patients of these patients. Finally, we compared the efficacy and safety between 28 patients in group A and 25 patients in group B (Figure 1). The characteristics of the patients in groups A and B are shown in table 1. Group A contained 11 females and 17 males, and group B contained 12 females and 13 males (p = 0.356). The median age was 55 (range, 33-68) years in group A, and 61 (43-68) years in group B (p = 0.056). The patients in group B tended to be older than those in group A. The median body weight was 60 (range, 46-85) kg in group A and 61 (41-90) kg in group B (p = 0.63). Previous IFN-based therapies were performed in 12 patients (43%) in group A and 16 (64%) in group B. Group A had nine relapsers and three non-responders, whereas group B had 12 relapsers and four non-responders. No significant differences in hemoglobin level, platelet count and white blood cell count were detected at baseline between groups A and B. The mean HCV-RNA level was 6.4 ± 0.6 log copies/mL in group A and 6.5 ± 0.6 log copies/mL in group B (p = 0.681). The IL28B and ITPA genotypes were examined in 45 (85%) and 34 (64%) patients, respectively. No differences in the distribution of the IL28B and ITPA genotypes were detected between groups A and B.
Baseline characteristics of patients with chronic hepatitis C virus infection allocated to the telaprevir (TVR) -standard and TVR-reduction groups.
| TVR-standard (group A) | TVR-reduction (group B) | p-value | |
|---|---|---|---|
| Patients (n) | 28 | 25 | |
| Male/Female | 18/10 | 13/12 | 0.356 |
| Median age (range) years | 55 (33-68) | 61 (43-68) | 0.056 |
| Body weight | 60 (46-85) | 61 (41-90) | 0.63 |
| Body mass index | 22.3 (17.7-29.8) | 23.6 (16.4-27.8) | 0.762 |
| Previous IFN therapy (+/−) | 12/16 | 16/9 | 0.124 |
| Relapser/non-responder | 9/3 | 12/4 | 1 |
| Fibrosis stage (F0/F1/F2/F3/ND) | 0/13/8/1/6 | 1/16/4/1/3 | 0.326 |
| IL28B genotype (major/non-major) | 18/4 | 16/7 | 0.34 |
| ITPA genotype (major/non-major) | 11/4 | 12/7 | 0.529 |
| HCV RNA (log copies/mL) | 6.4 ± 0.6 | 6.5 ± 0.6 | 0.681 |
| WBC (mL) | 5,436 ± 1,822 | 5,272 ± 1,595 | 0.624 |
| Hb (g/dL) | 14.6 ± 1.5 | 14.3 ± 1.0 | 0.412 |
| Plt (x 104/mL) | 17.3 ± 5.2 | 16.5 ± 4.8 | 0.515 |
| ALT (lU/L) | 60.8 ± 37.5 | 38.7 ± 17.1 | 0.036 |
| γ-GTP (lU/L) | 60.7 ± 57.6 | 65.0 ± 100.8 | 0.557 |
| Cre (mg/L) | 0.72 ± 0.15 | 0.71 ± 0.16 | 0.866 |
IFN: interferon. WBC: white blood cell. Hb: hemoglobin. Plt: platelet. ALT: alanine aminotransferase. γ-GTP: gamma-glutamyl transpeptidase. Cre: creatinine.
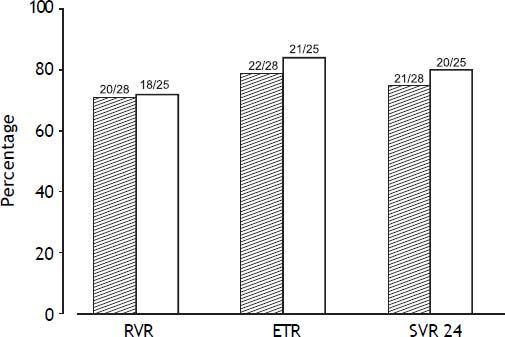
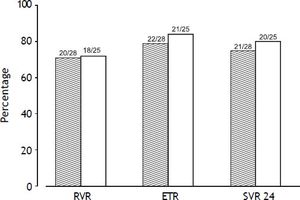
The RVR rate was 71% (20/28) in the TVR-standard group and 72% (18/25) in TVR-reduced group (p = 0.963). The ETR rate was 78.6% (22/28) in the TVR-standard group and 84% (21/25) in the TVR-reduced group (p = 0.614). The SVR24 rate was 75% (21/28) in the TVR-standard group and 80% (20/25) in the TVR-reduced group (p = 0.664). No significant difference in the treatment response was detected between the two groups (Figure 2). In one patient in the TVR-reduction group, the HCV RNA was reduced to below 1.2 log copies/mL at week 12 of treatment.
Viral response rates at week 4 (RVR), the end of treatment response (ETR), and week 24 after treatment (SVR24). Gray bars show the rates in the TVR-standard group, and white bars the rates in the TVR-reduced group. No significant difference in the viral response rate was detected between the two groups.
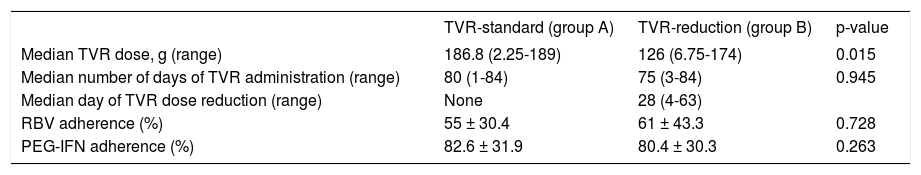
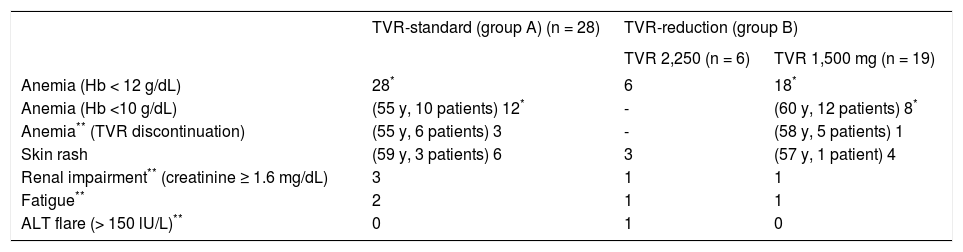
In the TVR-standard group, 14 (50%) patients discontinued TVR treatment and 4 (14%) patients stopped the combination therapy with PEG-IFN and RBV. In the TVR-reduced group, 14 (56%) patients discontinued TVR treatment, and 18 (72%) patients received reduced TVR doses (2250 mg reduced to 1,500 mg) between day 4 and day 63 of treatment (median, day 28). Five of the group B patients (20%) stopped combination therapy with PEG-IFN and RBV. The total TVR dose administrated was greater in group A than in group B (p = 0.015) (Table 2). TVR was discontinued for the following reasons in group A patients: skin rashes in six patients, renal impairment (creatinine ≥ 1.6 mg/dL) in three, anemia in three, and severe general fatigue in two patients; in group B patients: it was discontinued skin rashes in seven patients, renal impairment in two, severe general fatigue in two, and ALT elevation and anemia in one patient each (Table 3). No difference in the incidence of adverse events was detected between the two groups. However, renal impairment occurred more frequently during TVR 2,250 mg treatment (4/34; 11.7%) than during TVR 1,500 mg treatment (1/19; 5.2%), although the difference was not significant (p = 0.44). No patients in the present study died due to treatment-related adverse events.
Adherence to drug treatments.
| TVR-standard (group A) | TVR-reduction (group B) | p-value | |
|---|---|---|---|
| Median TVR dose, g (range) | 186.8 (2.25-189) | 126 (6.75-174) | 0.015 |
| Median number of days of TVR administration (range) | 80 (1-84) | 75 (3-84) | 0.945 |
| Median day of TVR dose reduction (range) | None | 28 (4-63) | |
| RBV adherence (%) | 55 ± 30.4 | 61 ± 43.3 | 0.728 |
| PEG-IFN adherence (%) | 82.6 ± 31.9 | 80.4 ± 30.3 | 0.263 |
RBV: ribavirin. PEG-IFN: pegylated interferon.
Adverse events necessary for TVR discontinuation or dose reduction.
| TVR-standard (group A) (n = 28) | TVR-reduction (group B) | ||
|---|---|---|---|
| TVR 2,250 (n = 6) | TVR 1,500 mg (n = 19) | ||
| Anemia (Hb < 12 g/dL) | 28* | 6 | 18* |
| Anemia (Hb <10 g/dL) | (55 y, 10 patients) 12* | - | (60 y, 12 patients) 8* |
| Anemia** (TVR discontinuation) | (55 y, 6 patients) 3 | - | (58 y, 5 patients) 1 |
| Skin rash | (59 y, 3 patients) 6 | 3 | (57 y, 1 patient) 4 |
| Renal impairment** (creatinine ≥ 1.6 mg/dL) | 3 | 1 | 1 |
| Fatigue** | 2 | 1 | 1 |
| ALT flare (> 150 lU/L)** | 0 | 1 | 0 |
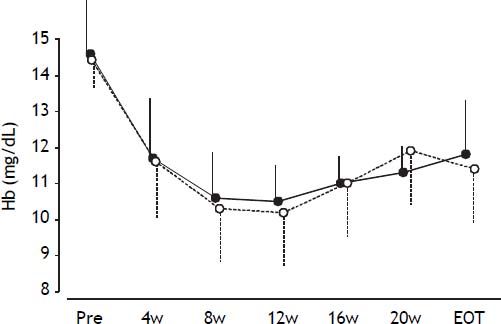
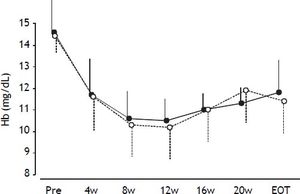
During treatment, the hemoglobin levels were reduced continuously in all patients (Figure 3), with no significant difference in hemoglobin levels at any time between groups A and B.
Mean hemoglobin levels during treatment. Error bars represent standard errors. Open circles with dotted lines represent hemoglobin levels in patients in the TVR-reduced group. Closed circles with solid lines represent hemoglobin levels in patients in the TVR-standard group. No significant differences in hemoglobin levels were detected at any time point between the two groups.
Four patients who maintained the initiated TVR and RBV doses achieved SVR. These patients consisted of two females and two males (three treatment-naïve and one relapser) in the age range 31-60 years, with body weights ranging from 54-85.5 kg, body mass index (BMI) ranging from 20.6-31.5, and baseline hemoglobin levels in the range 14.0-16.6 g/ dL. Three of the patients had the IL28B TT genotype and one had the non-TT genotype; three had the ITPA CC genotype and one had the non-CC genotype.
DiscussionDrug adherence is one of the most important factors in achieving SVR in patients treated with PEG-IFN and RBV.4,24 However, several adverse events require drug dose reductions. Compared to PEG-IFN and RBV therapy, TVR combined triple therapy induced a greater variety of adverse events with more frequency. The TVR dose is recommended to remain at 2,250 mg for 12 weeks, and should not be reduced to protect against the emergence of drug resistance to protease inhibitors. To our knowledge, no studies of TVR drug adjustment in response to adverse events have been reported. Therefore, we conducted this prospective randomized study.
In this study, no significant difference was observed between the TVR-standard and -reduced groups in terms of RVR, ETR, SVR24 and the rate of discontinuation of the study drugs in all cases. Several reports have evaluated the efficacy and safety in patients treated with 1,500 mg of TVR combined therapy. Suzuki, et al. reported that TVR 500 mg administered every 8 h (q8h) combined therapy achieved similar SVR rates to those obtained using standard therapy with TVR 750 mg q8h.25 Additionally, the SVR rates were identical in both the TVR 750 mg q12h combined and q8h therapies.26 Our results were consistent with their reports.
In our study, hemoglobin levels showed similar patterns in both groups, suggesting that TVR dose adjustments had the same effect on the improvement of anemia as do RBV dose adjustments. The incidence of skin rash and general fatigue in the TVR-standard group was similar to that in the TVR-reduced group. However, the incidence of renal impairment was lower in patients in the TVR-reduced group compared to those in the TVR-standard group. Sezaki, et al.27 reported that TVR 1,500 mg combined therapy reduced anemia, but not renal impairment, compared to TVR 2,250 mg therapy. In comparison, other studies showed that TVR 1,500 mg therapy reduced the incidence of renal impairment.25,26 The treatment protocol in our study differed from those used in other studies. First, the initial dose of TVR was 2,250 mg/day in our study. Second, the patients were treated with a clearance-based dosage of RBV. These differences in treatment protocol might have influenced the occurrence of adverse events.
No adverse effects, including anemia occurred in one of the TVR-maintained group and three of the TVR-reduced group. These four patients who maintained the initial doses of the three drugs achieved an SVR. It has been suggested that drug adherence is an important factor for SVR in TVR-combined therapy.13 However, there were few patients (4/55, 4%) in our study. By contrast, one patient in the TVR-reduced group showed HCV relapse after 24 weeks of combination therapy. The TVR dose in the triple therapy was determined by the dose-finding study, which showed that 2,250 mg of TVR achieved the greatest anti-HCV effect.28 However, it is not clear whether TVR 2,250 mg is necessary during triple combined-therapy for all patients such as older patients and those with lower body weight. Likewise, it is not known whether TVR 1,500 mg is sufficient to achieve an SVR in all patients with HCV. We propose that TVR dose adjustments should consider individual patient’s characteristics and adverse events.
This study had several limitations. First, the small sample sizes in each subgroup hampered our capacity to draw conclusions. Second, our study cohort was limited to Japanese CHC subjects with relatively low body weights or low BMI, who were infected predominantly with HCV genotype 1b, in contrast to patients in Western countries.29,30 Hence, our results cannot be extended to patients with HCV genotype 1a or those of other ethnicities. Third, the latest guideline for HCV treatment recommends sofosbuvir or simeprevir (SMV) combined with PEG-IFN plus RBV for HCV genotype 1, instead of TVR.31 Sofosbuvir combined with PEG-IFN plus RBV therapy is not widely available in Japan. SMV is a second-generation inhibitor of the NS3/4A serine protease, which does not induce the additional adverse events in patients treated with PEG-IFN combined triple therapy.32–34 However, TVR has an HCV drug resistance profile that differs from SMV.31 It has been speculated that TVR has a potential effect on SMV-resistant HCV, Q80R/K or D168A/V/T/ H.35 Thus, larger prospective studies with cohorts of various ethnicities will be needed to confirm these results.
ConclusionThe reduction of TVR dose to 1,500 mg prevented anemia progression without weakening the antiviral effect during triple therapy for patients with HCV. TVR dose adjustment might be an appropriate option for preventing adverse events.
Abbreviations- •
ALT: alanine aminotransferase.
- •
HCV: hepatitis C virus.
- •
RVR: rapid viral responders.
- •
ETR: end of treatment response.
- •
NVR: non-viral responders.
- •
PEG-IFN: pegylated interferon.
- •
SVR: sustained viral response.
In the past year, Dr. Akihiro Tamori has received research funding from MSD K.K. Dr. Dr. Norifumi Kawada has consulted for MSD K.K. and Chugai Pharmaceutical Co., Ltd. and has received research funding from MSD K.K. and Chugai Pharmaceutical Co., Ltd. The remaining authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
AcknowledgementsWe would like to thank Ms. Yoko Yasuhara, and Ms. Sanae Deguchi for collecting data.
The English in this document has been checked by at least two professional editors, both native speakers of English.