Despite steady progress in therapeutics of liver disease, portal systemic encephalopathy remains to be a great challenge for clinicians because of the heterogeneity of neuropsychiatric symptoms, multiple risk factors and complexity on achieving a sustained response. We aimed to evaluate the efficacy of L-Ornithin, L-Aspartate versus lactulose in Mexican patients with hyperammonemic hepatic encephalopathy. A total of 20 patients were randomly allocated to receive either lactulose (n = 10) or L-ornithine – L-aspartate (n = 10) for 2 weeks. At baseline, patients of both groups were comparable in age (64 ± 7 versus 60 ± 6) and degree of hepatic failure according to the Child-Pugh scale (9.2 ± 1.3 versus 9.2 ± 1.1). A significant decrease in ammonia levels was observed both in the lactulose group (120.4 ± 8.1 versus 91.4 ± 10, p < 0.05) and in the LOLA group (141.6 ± 9.1 versus 96.9 ± 9.3, p < 0.05). Moreover, in patients who received LOLA a significant improvement was observed in mental status (1.0 ± 0.14 versus 0.4 ± 0.16, p < 0.05), Number Connection Test (184 ± 43 versus 88 ± 7, p < 0.05), asterixis (14.6 ± 2.8 versus 6.7 ± 1.5, p < 0.05), as well as EEG findings (6.8 ± 0.6 versus 8.1 ± 0.2 cycles per second, p < 0.05). Compliance with study medications was similar between the lactulose group (94%) and the LOLA group (100%). No serious adverse events were reported in the two groups; however, in the lactulose group an increase in the number of weekly defecations was reported, as well as a higher incidence of abdominal pain or flatulence. Finally, both patient groups reported an improvement in the Visual Analogue Scale for EuroQol index (51.1 ± 24.1 versus 61.5 ± 15.8, p < 0.05, in the lactulose group; 56.5 ± 24.5 versus 70 ± 19.4, p < 0.05, in the LOLA group). In conclusion, oral administration of lactulose or L-ornithine - L-aspartate to Mexican patients with cirrhosis and hyperammonemic encephalopathy significantly reduced serum ammonia levels in study groups and additionally improved mental status parameters, number connection test, asterixis scores, and EEG activity in the group receiving L-ornithine-L-aspartate.
Hepatic encephalopathy is a well-recognized clinical complication of chronic liver disease. About 30% of patients with cirrhosis die in hepatic coma. The estimated incidence of hyperammonemic portal systemic encephalopathy is 26% at 5 years of cirrhosis diagnosis with a stepwise increase up to 70% in patients with poor liver function (Child-Pugh C). The main objective of therapy is to decrease intestinally derived toxins produced by excessive bacterial activity and increased formation of ammonia.1-3
Treatment for PSE is based on measures oriented to avoid the production and passage to the bloodstream of intestinal nitrogenous compounds, in general, and of ammonia, in particular.4-7 Traditionally, first choice therapeutic approach has been using of the «nonabsorbable» antibiotics neomycin, kanamycin sulfate, and paromomycin.8 Such antibiotic therapy might be effective, but absorption of a small fraction of these antibiotics caused ototoxic and nephrotoxic side effects; this treatment now is used infrequently. Metronidazole given in a 250-mg dose three or four times a day is as effective as oral neo-mycin and does not cause ototoxicity or nephrotoxicity. However, to avoid peripheral neuropathy, metronidazole therapy should not be extended beyond 2 weeks. More recently, rifaximin has demonstrated to be as good as lactulose9 or lactitol10,11 for treatment of patients with hepatic encephalopathy. However, long term use of antibiotics raises concerns about bacteria resistance and high therapeutic costs for affected patients.
Disaccharides such as lactulose or lactitol pass through the small bowel undigested. In the colon, bacteria degrade lactulose to various organic acids (eg, formic acid, acetic acid) with subsequent lowering of colonic pH. The mode of action is uncertain. It may involve bacteriostatic effects, cathartic effects, or enhancement of conversion of ammonia to ammonium with excess hydrogen ion. Presumably, ammonium is then excreted into the feces and eliminated.12,13 Lactulose remains the mainstay therapy, despite the paucity and inconsistency of clinical trials demonstrating its efficacy.14 The dose should be adjusted to accomplish three or four soft bowel movements each day. Lactulose can be given orally through a nasogastric tube or through retention enemas. The usual oral dose is 50 to 120 mL each day in divided doses. Stool pH should be below 6.0. Side effects include initial bloating and flatulence and then severe diarrhea with dehydration and hyperglycemia and acidosis if the dosage is too high.
Fundamental conceptual advances in our understanding of hepatic encephalopathy have confirmed the central role of ammonia in the pathogenesis of PSE.7 Consequently, our interest has been focused on therapeutic agents, such as L-ornithine-L-aspartate (LOLA), which provides critical substrates for both urea and glutamine synthesis, the key pathways of ammonia detoxification.15 LOLA, a stable salt of the natural L-amino acids ornithine and aspartic acid has been shown to decrease ammonia levels in animal experiments with hyperammonemia.16-18
In cirrhotic patients with portosystemic encephalopathy, LOLA has confirmed its efficacy both in uncontrolled clinical trials19,20 and more recently in well designed controlled clinical trials, versus placebo.21-24 It is important to mention that previous studies have been conducted in European countries or China using Caucasian or Oriental patients. However, no similar investigation has been performed in Latin American patients. It has been reported that ethnicity can effects therapeutic responses to anti-viral therapies25 and long term prognosis of patients with advanced liver diseases.26,27 In particular, we ignore if Latin American ethnic background affect the degree of effectiveness of patients receiving LOLA. Neither data is available on the relative efficacy of LOLA compared to lactulose administration which is considered as the first line of treatment in many countries including Mexico. Therefore, we decided to conduct this prospective controlled clinical trail to evaluate the safety and efficacy of LOLA versus lactulose administration to Mexican patients with hyperammonemic hepatic encephalopathy.
Materials and methodsThe study was carried on from May 04, 2004 to February 20, 2006 at the Medica Sur Clinic and Foundation located in the southern area of Mexico City. All subjects had advanced liver cirrhosis and were admitted to the study after signing the informed written consent form (ICF) which was properly explained to every patient and at least one relative of the volunteer. The protocol and ICF were approved by the Institutional Review Board and the conduction of the trial was in accordance with the revised Helsinki Declaration and ICH-Good Clinical Practices guidelines.
Patients. All adult eligible patients were enrolled in the study after been assessed to the following entry criteria: 1. Hepatic cirrhosis, regardless of the etiology, based on clinical, biochemical, histological and/or imaging data, who agree to participate in the study by signing an informed consent. 2. Patients with chronic persistent grade I or II overt hepatic encephalopathy in the last 6 months, present at the moment of the selection visit, according to the West Haven Criteria.28,29 4. Fasting plasma ammonia greater to 60 (μg/dL in the exams carried on during the selection visit.
Exclusion criteria included: 1. Patients with fist episode of acute encephalopathy. 2. Patients with unstable or severe hepatic encephalopathy, stage III or IV. 3. Patients with degenerative CNS disease or major psychiatric illness. 4. Gastrointestinal bleeding in the last 30 days. 4. Evidence of active infectious processes or antibiotic requirement in the preceding 30 days. 5. Serum creatinine higher than 1.8 mg/dL. 6. Hyponatremia (less than 130 mEq/L). 7. Pregnancy or breast-feeding, refusal to use a method of contraception in women of child-bearing age. 8. Decompensated or uncontrolled diabetes mellitus.
Additionally, the following findings were considered as elimination criteria: 1. Progression to Stage III or IV hepatic encephalopathy. 2. Complications due to portal hypertension, such as refractory ascitis or variceal hemorrhage. 3. Medication compliance less to 80%. 4. Development of serious active infectious processes requiring antibiotic administration (for example, spontaneous bacterial peritonitis).
Initial study Population: During the above mentioned period, a total of 78 cirrhotic patients were assessed in the pre-screening stage. However, 52 did not satisfy the eligibility criteria mainly due to hepatic encephalopathy associated to a recent esophageal variceal bleeding (n = 25) or infectious episode (n = 12), uncontrolled diabetes (n = 5), renal failure (n=5), Hyponatremia (n=5); of the 26 subjects who signed the informed consent form, 6 had to be excluded during screening due to the following reasons: 4 had ammonia levels within normal range, although levels had previously been elevated; 1 patient had decompensation caused by digestive bleeding; 1 patient declared to have previously used LOLA (though this had been denied in the selection visit).
Final randomized Study Population(Figure 1): A total of 20 patients with cirrhosis satisfied all entry criteria and were randomized to receive study medications.
Study medicationsL-ornithine - L-aspartate (Hepa-Merz Granules®, Merz Pharma, S.A, de CV.) was given as 5 g sachet formulation containing granules (3 g L-ornithine - L-aspartate). Dosing: 9 g x day for 2 weeks (1 sachet, 3 g/3 times daily). Doses could be adjusted to a maximum of 18 g x day (2 sachets containing 3 g/3 times daily) if patients required it in accordance the Researcher’s opinion.
Lactulose (Regulact®, ICN Farmacéutica) was given as a syrup formulation containing 67 g lactulose/100 mL; Dosing: 30 mL x day for 2 weeks (10 mL/3 times daily). Doses could be adjusted to a maximum of 60 mL x day (20 mL/3 times daily) if patients required it in the Researcher’s opinion.
• Handling and assortment of study drugs.
Study drugs were stored in a Research Pharmacy in separated locked containers. Each container was identified by a separate label, in order to permit a close control. Drugs were labeled including generic and commercial names, manufacturing laboratory, formulation, dose, batch number, production date and expiration date. The exact quantity of drugs dispensed to study subject was properly registered. Drugs were given to study subjects on a weekly basis, during each visit to the Research Center.
• Administration and drug accountability.
Study drugs were randomly assigned by the Pharmacy staff using a pre-designed randomization Table. Subjects were instructed to hand back unused study drug (either lactulose flasks or Hepa-Merz® sachets). Quantitative assessment of the compliance degree percentage was performed for each patient.
• Previous and concomitant medications.
The most recent previous medications for hepatic encephalopathy were lactulose (n = 12), antibiotics (n = 6), or low protein diets (n = 2). All the patients accepted to withdrawn previous medication during one week before randomization to the study. Most subjects received 2-3 concomitant medications for gastric protection (ranitidine, omeprazole, or pantoprazole) or diuretics (spironolactone o furosemide). There was no difference in the number or main dosing schedules between the two study groups.
• Standardized Diet.
All admitted subjects received a standardized 1,727 Kcal diet, which included 76 grams of protein (equivalent to 1.0 to 1.2 g per kg bw, per day) - two thirds were vegetable proteins, and one third was animal source protein. This diet program was followed for 3 weeks (1 week previous to randomization (homogenization week) and 2 weeks during study period. On each weekly visit, subjects were strongly motivated to follow the recommended standardized diet.
• Blood Chemistry (Safety parameters).
Blood samples were taken from all participating subjects at baseline, in order to perform the following tests: Hematology, liver function test, glucose, creatinine, serum electrolytes and plasma ammonia levels; a urine analysis was also performed. Subsequently, samples were repeated on study days 7 and 14 and sent to the Medica Sur Clinic and Foundation Pathology Department (CAP and ISO 9001-2000, certified) for blind analysis (all laboratory test were subject to constant quality control by means of suitable method checks).
Therapeutic endpointsAll patients were assessed to determine the index of hepatic encephalopathy at baseline and furthermore on a weekly basis, including the following tests.
Mental state. Grading was done according to a scale from 0 to 4+ (from normal to deep coma), in accordance with the West Haven criteria described above.29
Number connection test. The NCT is the time in seconds that a patient requires to connect 25 circled numbers; it is also expressed in grades. To overcome the learning curve effect, four different versions of the test were used.
Asterixis. Flapping was graded semiquantitatively as follows: grade 0 meant no flapping motions; grade 1+ rare flaps (up to 5 flaps/min); grade 2+, occasional irregular flaps (6-10 flaps/min); grade 3+, frequent flaps (11-20 flaps/min); and grade 4+, almost continuous flapping or patient to ill to perform the test.
Blood ammonia. Fasting venous plasma ammonia levels were measured with a Beckman microtitrator and using the enzymatic determination with the glutamate dehydrogenase test. Blood samples were sent to the Laboratory in refrigerated transport within 15 minutes of withdrawal. Normal values at our Laboratory were those below 70 μg/mL. A semiquantitative grading scale (0 to 4+) was used to include this variable in the PSE index.
Electroencephalograms. EEGs were taken with a Van Gogh EPB machine, with the standard 20 electrode placement technique. EEGs were blindly interpreted and graded from 1+ to 4+ by two od the study authors (SAC, GGR) who were blinded to the medication administered to patients.
Portal-Systemic encephalopathy index (PSEI). Each variable was weighed in accordance with its importance: mental state was assigned a factor of 3, and each of the other variables a factor of 1. Each variable was graded on a scale from 0 to 4+. The total weight of the score is called PSE sum. Considering that the maximum PSE score is 28, the PSEsum/maximum PSE ratio represents the PSE Index, which arbitrarily reflects the changes occurring during the study period.29
Bowel movements and GI adverse Events. All patients were instructed to fill a diary to register the number of bowel movements and the appearance of side effects with particular attention to any gastrointestinal symptoms.
Quality of life assessment. All patients filled the Euro-Qol Index survey, including the visual analog scale evaluation at baseline and at 7 and 14 days evaluations. We also incorporated the nonutility-based Short Form-36v2 survey, which provides a detailed profile of health related quality of life.30
Statistical analysis and sample size estimationThe statistical evaluation was performed using a SPSS Statistical software version 6.12 for Windows. Descriptive statistics included mean, standard deviation or error standard. A paired or un-paired t Test was used to compare means before and after study medication administration. Values lower than 5% were considered as significant. Sample size calculation was based on the following assumptions: (1) Mean expected baseline ammonia level of 154.4 μg/dL;21 (2) Ammonia standard deviation 58.5. (3) Expected ammonia reduction rate higher than 30% (delta); (4). Alpha error of 5%; (5). An accepted beta error of 20%. The final number of patients required, using the Dawson formula (N = 2 Sx2 (Zα - Zβ) 2 / Δ2 or N = 2 [(Z± - Z/β) σx/μ1 - μ2])31 was 20 patients.
ResultsEnrollment report. The Study included a total of 78 patients with hepatic cirrhosis that at some point during their clinical evolution had experienced signs and symptoms of hepatic encephalopathy. Of these patients, 52 reported an acute triggering event (hemorrhage, infections, other co-morbidities) considered as reason enough for protocol exclusion.
Final Study Population:
A total of 20 cirrhotic patients with chronic persistent overt hepatic encephalopathy satisfied all of the eligibility criteria. Baseline epidemiological findings showing the comparability between the two study groups are described in Table I.
Basal clinical characteristics.
| Parameter | Lactulose group (n = 10) | LOLA group (n = 10) | p Value |
|---|---|---|---|
| Age (years) | 64 ± 7 | 60 ± 6 | NS |
| Gender (F/M) | (8:2) | (10:0) | NS |
| Cirrhosis type | |||
| C virus | 4 | 5 | |
| Autoimmune | 2 | 1 | |
| PBC | 0 | 1 | |
| Idiopathic | 4 | 3 | |
| Evolution of cirrhosis (months) | 22 (4-63) | 31 (3-144) | NS |
| Evolution of PSE (months) | 11 (6-60) | 23 (6-96) | NS |
Values are expressed as mean ± SD
Clinical findings(Table II). There were no differences between the groups in clinical parameters as observed in physical exam or laboratory and complementary exams.
Baseline clinical findings.
| Parameters | Lactulose group (n = 10) | LOLA group (n = 10) |
|---|---|---|
| BMI (Body Mass Index) | ||
| weight/height2 | 27.0 ± 4.9 | 28.7 ± 3.7 |
| SBP (mm/Hg) | 112 ± 11 | 112 ± 10 |
| DBP (mm/Hg) | ||
| HR (beats/minute) | 68 ± 5 | 73 ± 8 |
| Clinical findings | 69 ± 5 | 71 ± 10 |
| Ascitis | 4 | 5 |
| Encephalopathy | 10 | 10 |
| Esophageal varices | 7 | 8 |
| Child-Pugh score | 9.2 ± 1.3 | 9.2 ± 1.1 |
Values are expressed as mean ± SD
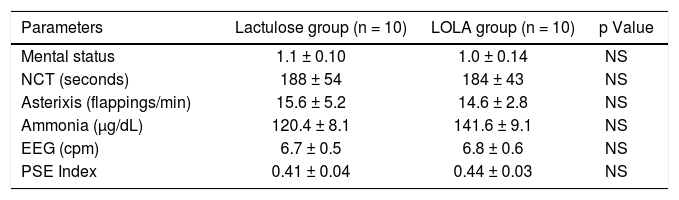
Baseline degree of encephalopathy(Table III). There were no differences between the groups in parameters reflecting the degree of hepatic encephalopathy at baseline.
Hepatic encephalopathy parameters.
| Parameters | Lactulose group (n = 10) | LOLA group (n = 10) | p Value |
|---|---|---|---|
| Mental status | 1.1 ± 0.10 | 1.0 ± 0.14 | NS |
| NCT (seconds) | 188 ± 54 | 184 ± 43 | NS |
| Asterixis (flappings/min) | 15.6 ± 5.2 | 14.6 ± 2.8 | NS |
| Ammonia (µg/dL) | 120.4 ± 8.1 | 141.6 ± 9.1 | NS |
| EEG (cpm) | 6.7 ± 0.5 | 6.8 ± 0.6 | NS |
| PSE Index | 0.41 ± 0.04 | 0.44 ± 0.03 | NS |
Values are expressed as mean ± SD
Baseline biochemical findings(Table IV). In order to assess comparability between groups regarding the degree of systemic impairment, hepatic function impairment, and serum sodium levels, each one of these parameters was accurately evaluated within both groups; no significant differences were observed.
Baseline biochemical parameters.
| Parameter | Lactulose group (n = 10) | LOLA group (n = 10) | p value |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.0 ± 1.2 | 12.4 ± 2.1 | NS |
| White blood cells (x103 x mm3) | 4.7 ± 1.1 | 4.4 ± 1.8 | NS |
| Platelets (x103 x mm3) | 107 ± 54 | 88 ± 47 | NS |
| Total bilirubin (mg/dL) | 3.9 ± 2.8 | 3.3 ± 1.7 | NS |
| Albumin (g/dL) | 2.8 ± 0.4 | 2.7 ± 0.4 | NS |
| Prothrombine time (seconds) | 12.2 ± 3.5 | 13.7 ± 2.7 | NS |
| Serum sodium levels (mEq/L) | 135 ± 11 | 140 ± 8 | NS |
Values are expressed as mean ± SD. Student’s t-Test
Efficacy assessment.Figure 2, and (Table V) shows the most relevant study results focusing on PSE index parameters at baseline, and after one and two weeks of treatment. As it may be seen in the Table, patients treated with lactulose achieved a significant decrease in blood ammonia levels, but had no improvement in the other parameters nor in the PSE index. On the other hand, cirrhotic patients treated with LOLA achieved a significant decrease not only in serum ammonia levels, but also in time required to perform the Number Connection Test, in the degree of asterixis, as well as a significant improvement in the number of cycles per second in EEG activity. Consequently, a statistically significant improvement in PSE index could be observed in the intra-group analysis. No significant differences could be observed in the inter-group statistical analysis.
Effect of Lactulose and LOLA on PSE parameters.
| Parameter | Lactulose group | LOLA group | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 1 | Week 2 | Baseline | Week 1 | Week 2 | |
| Mental status | ||||||
| Stage 0 | 0 | 0 | 5 | 0 | 5 | 6 |
| Stage 1 | 9 | 10 | 4 | 9 | 5 | 4 |
| Stage 2 | 1 | 0 | 1 | 1 | 0 | 0 |
| Mean | 1.1±0.10 | 0.8±0.20 | 0.6±0.22 | 1.0±0.14 | 0.5±0.17 | 0.4±0.16* |
| NCT (seconds) | 188±54 | 177±34 | 155±49 | 184±43 | 135±27 | 88±7* |
| Asterixis (flp/min) | 15.6±5.2 | 12.3±3.8 | 13.8±4.2 | 14.6±2.8 | 12.0±2.4 | 6.7±1.5* |
| Ammonia (μg/dL) | 120.4±8.1 | 96.9±1 8 | 91.4±10* | 141.6±9.1 | 109.7±9.5 | 96.9±9.3* |
| EEG (cps) | 6.7±0.5 | NP | 6.9±0.4 | 6.8±0.6 | NP | 8.1±0.2* |
| PSE Index | 0.41±0.04 | NA | 0.35±0.05 | 0.44±0.03 | NA | 0.28±0.04* |
Effect of treatment on quality of life indicators(Table VI). A non significant improvement was observed in SF-36 Scale scores both in the group of patients receiving lactulose and in the group receiving LOLA. The Functional Health Subscale and the Mental Health Subscale were also assessed, and no statistically significant difference was reported; however, a trend towards improvement could be observed in the LOLA group.
Effect of Lactulose and LOLA treatments on quality of life indicators.
| Parameter | Lactulose group | LOLA group | ||
|---|---|---|---|---|
| Baseline | final | Baseline | final | |
| SF-36 score | 42.0 ± 19.5 | 41.0 ± 15.3 | 47.0 ± 22.0 | 54.0 ± 21.0 |
| • Physical health subscale | 40.0 ± 16.7 | 40.0 ± 16.9 | 47.0 ± 20.0 | 53.0 ± 21.0 |
| • Mental health subscale | 41.0 ± 21.1 | 39.0 ± 14.5 | 46.0 ± 23.0 | 52.0 ± 20.0 |
| EuroQoL visual analog scale | 51.1 ± 24.1 | 61.5 ± 15.8a | 56.5 ± 24.5 | 70.0 ± 19.4ab |
Values are expressed as Mean ± SD;
As far as the Visual Analogue Scale of the EuroQol Survey is concerned, there was significant improvement both in the lactulose group and in the LOLA group, as may be seen in Table VI. Also, there was a significantly greater improvement in the LOLA group if compared to the improvement in the lactulose group.
Study medication compliancePharmacy Staff calculated global adherence to treatment in both patient groups. Patients in the group treated with lactulose had a 94% compliance, while patients in the group treated with LOLA had a 100% compliance during the 2 weeks of treatment.
Adverse eventsDuring this study, 2 serious adverse events occurred, in two subjects; these events were reported to the Institutional Review Board. The first event occurred in a female subject, 53 years old (Subject CAB), who signed the informed consent form and was started on the one week homogenization phase with a standard diet. On the third day of the homogenization week she had a fatal digestive hemorrhage. This patient had not been assigned to any of the two therapeutic arms. The second adverse event occurred in a 72 year-old female subject (Subject QAY), who had been admitted to the study and randomly assigned to receive lactulose. This subject completed the 2 weeks treatment period without showing improvement in PSE parameters. One day after study conclusion she was moved to her hometown, in the state of Campeche located in the south of the Mexican Republic, but unfortunately she died of refractory septic shock, according to her relatives.
Digestive manifestations. Due to the well known mechanism of action of disaccharides a clear increase in weekly (week 1 versus week 2) bowel movements was observed in the Lactulose-treated group (12.4 ± 5.4 versus 15.4 ± 6.5) compared to a stable number of bowel movements in LOLA-treated group (15.4 ± 6.4 versus 14.7 ± 4.6). Abdominal pain daily frequency (28 ± 19 versus 45 ± 34) and flatulence (26 ± 15 versus 47 ± 31) were also increased in Lactulose-treated patients. LOLA-treated patients did not report increase abdominal pain frequency (23 ± 18 versus 25 ± 20) or flatulence (34 ± 18 versus 27 ± 16).
DiscussionThis study was an open label, randomized, clinical trial, in conformance with Good Clinical Practices, to assess the efficacy and safety of oral administration, for 2 weeks, of a lactulose syrup (Regulact®, ICN Laboratories) or granulate sachets containing 3 g of L-ornithine-L-aspartate (Hepa-Merz®, Merz Pharma), in 20 Mexican subjects with hepatic cirrhosis and hyperammonemia.
Traditionally, disaccharides as lactulose or lactitol, are the most commonly used therapeutics for hepatic encephalopathy. Despite the relatively inconsistency in results and the high frequency of side effects,14 lactulose is still considered as the first-line pharmacological treatment for hepatic encephalopathy based on the American College of Gastroenterology guidelines.6
L-ornithine-L-aspartate (LOLA), a stable salt of the natural L-amino acids ornithine and aspartic acid, has been shown to improve hepatic encephalopathy by: (1) stimulation of hepatic synthesis of urea (2) by increasing glutamine production in the muscle; (3) by regulating the relationship between branched and aromatic amino acids.16,18 It is important to remember that LOLA site of action is the liver or the muscle and therefore its role is focused on decreasing the high blood ammonia concentrations. On the contrary, disaccharides site of action is located to the intestinal area where colonic fermentation, by acidifying the colonic pH, impairs ammonia absorption.13
L-ornithine-L-aspartate has demonstrated beneficial therapeutic effects in several clinical studies either per oral22 or intravenous administration,19 compared to placebo administration. Successful results have also been demonstrated in cirrhotic patients with transjugular intrahepatic portosystemic shunts (TIPS).23 However, no previous trial has compared the effect of LOLA versus lactulose in a randomized controlled study.
To our knowledge, this is the first clinical trial reporting the safety and efficacy of LOLA-treatment versus lactulose-treatment for the short term management of hepatic encephalopathy. Patients randomized to both therapeutic groups showed a significant decrease in serum ammonia levels. However, only patients in the LOLA-treatment group had a significant improvement in Mental Status, Number Connection Test, asterixis, and EEG activity, and consequently a significant global improvement in the portosystemic encephalopathy index. Furthermore, patients treated with both study drugs reported an improvement in quality of life, according to the Visual AnalogueScaleoftheEuroQolSurvey. This improvement was significantly greater in patients who received L-ornithine-L-aspartate.
No data is available upon whether ethnic background affects the effectiveness of LOLA-compound. These results, conducted with Mexican patients, suggest that ethnic difference (based on Hispanic or Latino background of the participant patients) do not appear to affect the efficacy of LOLA-administration. Although the present study may be considered with the limitation of being an open label design, the global results showed a clear beneficial effect in both treatment groups with a clear trend to superiority in LOLA-receiving patients.
Finally, in terms of safety related to gastrointestinal adverse events, patients treated with L-ornithine - L-as-partate did not show a significant increase in number of weekly defecations, “intense” pain or flatulence, compared to the Lactulose group (in which these alterations were clearly observed).
The identification and correction of factors precipitating hyperammonemia must be the main concern for appropriate and rationale management of hepatic encephalopathy (HE), because the correction of those factors usually results in clinical improvement. In our study we eliminated reasonably the most frequent triggering factors such as digestive bleeding or sepsis. We restricted our study population to subjects having chronic, persistent HE.
In relation to the amount of protein administration, a recent trial has shown that diets with normal protein content can be administered safely to cirrhotic patients with episodic acute hepatic encephalopathy.32 However, in clinical practice many cirrhotic patient with chronic persistent HE remain with poor tolerance to high protein diets and therefore a transient reduction must be considered as a reasonable strategy for a short period of treatment. Prolonged restriction of dietary protein contributes to malnutrition of patients with cirrhosis and impairs the muscle ability to detoxify ammonia by glutamine synthesis.7 Vegetable proteins may be superior to meat protein, and the fiber content in diet appears to be beneficial.29 It is possible that the metabolic abnormalities seen in episodic acute encephalopathy differ from those seen in chronic persistent HE.33 Episodic acute HE is mainly related to occult triggering factors that improved despite the amount of proteins in the diet. Some studies have evaluated the positive effect of acute administration of LOLA during different proteins loads demonstrating a lower increased ammonia after 0.5 g per kg protein ingestion.19 The potential effect of chronic LOLA-administration to improve different proteins loads tolerance remains to be evaluated.
The ultimate therapy for hepatic encephalopathy is orthotopic liver transplantation. However, this approach is not always available in many Latin American or Asian Pacific countries were liver transplant programs are undeveloped.
In conclusion, L-Ornithine-L-Aspartate administration is safe and effective in reducing hyperammonemic hepatic encephalopathy in Mexican patients with hepatic cirrhosis.