Published incidences of hepatocellular carcinoma in the Black population of sub-Saharan Africa underestimate the true incidence of the tumor because of the many instances in which hepatocellular carcinoma is either not definitively diagnosed or is not recorded in a cancer registry. Despite this, it is manifestly evident that the tumor occurs commonly and is a major cause of cancer deaths in Black African peoples living in the sub-continent, particularly in those living in rural areas. 46,000 new cases of hepatocellular carcinoma have been recorded to be diagnosed in sub-Saharan Africa each year, and age-standardized incidences of the tumor as high as 41.2/100,000 persons/year have been documented. The highest incidence of hepatocellular carcinoma has been recorded in Mozambique. The tumor occurs at a young age in rural dwelling and, to a lesser extent, urban dwelling Black Africans. It is also more common in men than women, particularly in the younger patients. Cirrhosis co-exists with hepatocellular carcinoma in about 60% of patients and is equally common in the two sexes. The tumor is not only common in the Black African population, it also carries an especially grave prognosis, with about 93% of the patients dying within 12 months of the onset of symptoms. Caucasians living in the sub-continent have a low incidence of hepatocellular carcinoma and it occurs at an older age.
Africa is the second largest of the world’s continents. It covers 30,300,000 square kilometers, approximately one-fifth of the global land area. The continent is sparsely inhabited, with a population of approximately 800 million people accounting for only 12% of the global population.1 Most of Africa’s population lives to the south of the Sahara desert, where the vast majority of the inhabitants are Blacks. North Africa, in climate, customs, and cultural background, is Mediterranean rather than African, and many of the distinctive features of cancer in Africa belong to sub-Saharan Africa. None more so than hepatocellular carcinoma (HCC).
The global cancer burden has been, and will continue to be, increasingly influenced by the growth and aging of the world’s population. In the year 2000, 10.1 million new cases of cancer were recorded worldwide, and this figure is expected to increase to 15 million by the year 2020.2 Furthermore, by the year 2030 an annual total of 27 million new cases of cancer, 17 million deaths attributed to cancer, and 75 million people living with cancer could be expec-ted.3 The impact of these increases will be felt in all geographical regions, but especially in the economically-constrained regions, including sub-Saharan Africa.
Relevant Background InformationInformation on the epidemiology of HCC in sub-Saharan Africa, in common with that on all cancers in the sub-continent, has to date been neither complete nor always accurate.1,4–6 As a result, the true incidence of HCC in sub-Saharan Africa has been significantly underestimated. Knowledge of cancer incidence and patterns in the sub-continent has, until quite recently, been based largely on the work of a few pioneering clinicians and pathologists, who described series of cancer patients seen in their practices in terms of age, sex, cancer site, and histolo-gy.4–6 In such case series, comparisons based on relative frequencies of different cancers are known to be misleading.1
HCC occurs with high incidences in the Black African populations living in sub-Saharan Africa, but with a uniformly low incidence in the Caucasian populations.7 In the Black populations the tumor is far more common in rural than in urban dwellers.7 Necropsies and, to a lesser extent, liver biopsies are seldom performed in rural areas in Black African patients thought to be suffering from HCC. Estimates of the occurrence of the tumor in the sub-continent, but particularly in rural areas, where 80% of the Black population lives, therefore inevitably underestimate the true incidence of the tumor. Medical facilities in these rural areas are few and far between, and attendance at the local hospital is known to decrease with its distance from the patient’s home.8 Furthermore, HCC has in the past been, and even now is, often diagnosed solely on the basis of compatible clinical features, supported in some patients by either a raised serum a-fetoprotein concentration or the finding of a mass-lesion in the liver on conventional ultrasonographic imaging of the liver.9 The more sophisticated and expensive dynamic hepatic imaging techniques that today enable a firm diagnosis of HCC to be made based on the early hyper-vascularity of the tumor followed by rapid wash-out of the indicator from the tumor, are available in only the best equipped medical centers in the large cities in sub-Saharan Africa, and certainly not in the rural areas.
Reports on hospital-based series of patients with HCC in the African sub-continent have been, and remain, biased by the interests and competence of the local clinicians, the diagnostic facilities and therapeutic options available, and the accuracy and completeness of record keeping.1,5 Incidence rates, the appropriate statistic for comparing cancer risks between populations, should be derived from population-based cancer registries, which aim to record information on all new cases of cancer that occur in a defined population.1 Accurate registration depends upon identifying every case of the cancer, which can only be achieved if patients have ready access to the necessary health services. When the required resources are inadequate, the number of patients with access to these services is limited.
Another difficulty in sub-Saharan Africa is to correctly identify the place of residence of cancer patients, so that when incidence rates are calculated, only those cases belonging to the population at risk are included.1,10 Unfortunately, place of residence is not an obvious concept in some sub-Saharan African regions.10 This is especially true in eastern and southern Africa, where Blacks living and working in urban areas often retain a foothold in their home village, to which they return intermittently on vacation, to seek medical advice or care from local traditional healers (witch-doctors), or, when seriously ill or very old, to die.10 Consequently, they may not be included in either an urban-based or a rural-based cancer registry. Moreover, many Black Africans living permanently in rural areas rely on the diagnostic and treatment services of traditional healers rather than conventional doctors10 and they too would not be included in rural-based cancer registries. On the other hand, some Black men and women living in rural areas travel to towns or cities to stay with a relative when they are ill and need to visit a hospital. The address given to the hospital will be that of the urban relative. The few cancer registries in sub-Saharan Africa are almost all based in urban centers where diagnostic and treatment facilities are available. In compiling cancer registries accurately the main technical problem is to avoid registration of these ‘temporary’ residents visiting the urban area for short-term treatment or other reasons.10
In studying time trends in the occurrence of a tumor, the degree of completeness of registration of incident cancer cases should be constant throughout the period under consideration.11 This has not always been the case in the few such studies performed in sub-Saharan Africa. Compounding these difficulties, may be a low standard of literacy of the rural Black African population.10
Without a comprehensive and accurate population survey, it is not possible to ascertain the true incidence of HCC in a rural Black African population. One can only guess at how well the profile obtained from urban areas, if this has been undertaken, reflects that of the Black population as a whole.12
A further difficulty in recording accurate information about the incidence of HCC in sub-Saharan Africa is the lack of appropriately trained person-nel.1 There has been and continues to be a shortage, not only of epidemiologists and statisticians to plan and execute the collection of data in a processable form, but also of suitably trained personnel to man the registry infrastructure. Moreover, health ministries in most sub-Saharan African countries, when apportioning their limited financial and other resources, have regarded cancer as a lower public health priority than infectious and nutritional diseases.1 As a result, adequate governmental funds have not been, and continue not to be, made available for the institution and running of cancer registries.
With regard to mortality data, the information available in sub-Saharan Africa is even more questionable than that for incidence. Death registration and certification of cause of death are a statutory requirement for this information, but they are feasible in very few countries in the sub-continent.1
As a result of these hurdles and shortcomings, the history of cancer registration in sub-Saharan Africa from the time that the earliest registries were instituted in the 1950s has been disappointing. Even today, a minority only of countries have reliable cancer registries and, with few exceptions, these registries cover only the urban populations.
These deficiencies have been compounded by the infrequency and unreliability of population censuses in the African subcontinent.1
Despite the under-diagnosis and under-reporting of HCC in the African sub-continent, it is manifestly evident from the information available that the tumor occurs commonly and is a major cause of cancer deaths in Black African peoples living in sub-Saharan Africa.
Incidence of Hepatocellular CarcinomaThe International Statistical Classification of Diseases and Related Health Problems Code (ICD) in current use does not distinguish between HCC and the other primary cancers of the liver. In regions with a high incidence of HCC, including sub-Saharan Africa, this tumor accounts for 90 to 95% of all primary malignant tumors of the liver, whereas in regions with a low or intermediate incidence it accounts for 70 to 85% of these tumors.4,13–15 The only exceptions to this pattern of occurrence are in northeastern Thailand and parts of the Philippines, where the high incidence of cholangiocarcinoma as a result of endemic infestation of the liver with the flukes, Clonorchis sinensis or Opisthorchis viverini, is responsible for this tumor being the most common hepatic malignancy.
Allowing for the limitations in ascertaining the true incidence of HCC in the indigenous populations of sub-Saharan Africa, the information available should be considered in perspective with the global incidence of the tumor.
Global incidence of hepatocellular carcinomaBased on the number of new cases of cancer in humans each year, HCC is the sixth most common cancer worldwide.16 the fifth in males and the seventh in females.17 During the recent time that the number of new cases of HCC worldwide each year has been recorded, this number has increased year by year. In the most recently reported year, 748,000 new cases of the tumor were recorded, constituting 9.2% of all new cancers.17 Of these, 522,000 were in men and 226,000 in women, giving a sex ratio of 2.3:1.0.17 The total annual death rate from HCC in that year was 695,900:478,300 among men (the second most common cause of death from cancer) and 217,600 among women (the sixth most common cause of death from cancer).17 Of the patients developing HCC during that year, 93% died from the efffects of the tumor within 12 months of the onset of symptoms. This annual fatality ratio (0.93) is the highest of any human tumor.
HCC does not have a uniform world-wide distribution. Rather, of all the new cases of cancer recorded during recent years, the vast majority have occurred in resource-constrained regions.
Incidence of hepatocellular carcinoma in resource-constrained regionsDuring the year in question, 626,700 new cases of HCC were recorded in resource-constrained regions: 440,700 in men (third in cancer incidence) and 186,000 in women (sixth in cancer incidence) (male:female ratio 2.4:1.0).17 The number of deaths during the year was 580,600, and the 12-month fatality ratio 0.93:402,900 deaths in men (the second most common cause of cancer deaths) and 177,700 in women (the fifth most common cause of cancer deaths).17
As disturbing as these numbers undoubtedly are, they under-estimate the true incidence of, and the death rate from, HCC in resource-constrained regions. Based on published incidences, it is evident that 84% of all global cases of the tumor occur in economically-constrained regions, with 55% occurring in China alone.17,18 Resource-rich regions have a low incidence of HCC. Exceptions to this incidence pattern are Japan, a resource-rich country which has a high incidence of the cancer, and southern Europe, a resource-rich region with an intermediate incidence.19,20 The incidence of HCC varies by as much as 100-fold between high-risk and low-risk regions, a ratio that is among the highest for any of the major cancers.
An age-standardized incidence of greater than 15 per 100,000 of the population per year is regarded as a high occurrence of HCC. This incidence of the tumor in China is 52.1 per 100,000 persons/year and in Melanesia 25.0 per 100,000/persons/year.1 By comparison, the incidence of HCC is less than 5/100,000 persons/year in almost all resource-rich regions, with the tumor accounting for less than 1% of all malignant diseases.1
Incidence of hepatocellular carcinoma in sub-Saharan AfricaHCC was initially reported to occur in sub-Saharan Africa in 1879,21 although the first report of the high incidence of the tumor in Black Africans living in the subcontinent was published only in 1921.22 Since those early reports, a great deal has been learnt about the occurrence of HCC in sub-Saharan Black Africans, and it is now known that all Black ethnic groups throughout the sub-continent are affected by the tumor, although not to the same extent.
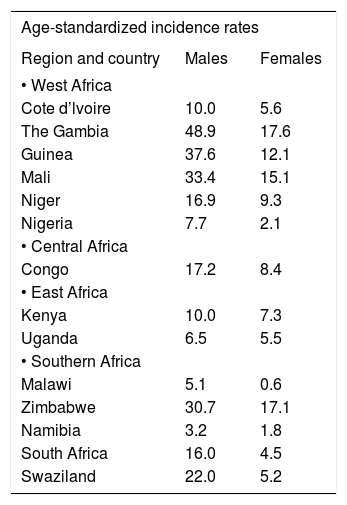
Published incidences of HCC in sub-Saharan Africa underestimate its true incidence because of the many instances in which the tumor is either not definitively diagnosed or is not recorded in a cancer registry. In recent times, 46,000 new cases of HCC have been recorded as being diagnosed in sub-Saharan Africa each year.1 Because the recorded annual occurrence rate of the tumor in sub-Saharan Black Africans is almost the same as its annual fatality rate, the annual occurrence figure at any one time is virtually the same as the total incidence of the tumor in the population at that time. The age-standardized incidence of the tumor in regions of sub-Saharan Africa are 41.2/100,000/persons/year in middle Africa, 29.7/100,000 persons/year in eastern Africa, and 20.9/100,000 persons/year in western Africa.1 Age-standardized incidence rates and relative (proportional) frequencies of the tumor in specific countries, documented in the International Agency for Research In Cancer (IARC) registry in 2003, are listed in tables 1 and 2. Of the six West African countries for which incidences are recorded in this analysis, four have high incidences and two intermediate incidences of HCC, the one Central African country included has a high incidence; three of the five southern African countries (South Africa and its immediate neighbouring countries) have high incidences, one has an intermediate incidence, and one a low incidence; and the two East African countries have intermediate incidences.19 Mozambique and many other countries in the sub-continent are not included in the tables because recent cancer registry figures are not available for these countries.
Age-standardized incidence rates of primary liver cancer in African by region.
| Age-standardized incidence rates | ||
|---|---|---|
| Region and country | Males | Females |
| • West Africa | ||
| Cote d’lvoire | 10.0 | 5.6 |
| The Gambia | 48.9 | 17.6 |
| Guinea | 37.6 | 12.1 |
| Mali | 33.4 | 15.1 |
| Niger | 16.9 | 9.3 |
| Nigeria | 7.7 | 2.1 |
| • Central Africa | ||
| Congo | 17.2 | 8.4 |
| • East Africa | ||
| Kenya | 10.0 | 7.3 |
| Uganda | 6.5 | 5.5 |
| • Southern Africa | ||
| Malawi | 5.1 | 0.6 |
| Zimbabwe | 30.7 | 17.1 |
| Namibia | 3.2 | 1.8 |
| South Africa | 16.0 | 4.5 |
| Swaziland | 22.0 | 5.2 |
Relative (proportional) frequencies of primary liver cancer in African countries by region.
| Relative (proportional) frequencies | ||
|---|---|---|
| Region and country | Males | Females |
| • West Africa | ||
| Burkino Faso | 25.9 | 7.5 |
| Cote d’lvoire | 14.6 | 4.2 |
| The Gambia | 58.5 | 19.1 |
| Guinea | 40.4 | 9.9 |
| Mali | 36.2 | 15.1 |
| Niger | 22.5 | 7.4 |
| Nigeria | 11.6 | 3.0 |
| • Central Africa | ||
| Cameroon | 38.2 | 10.3 |
| Congo | 26.4 | 8.9 |
| • East Africa | ||
| Kenya | 6.3 | 4.4 |
| Rwanda | 20.5 | 8.7 |
| Tanzania | 8.8 | 4.1 |
| Uganda | 3.6 | 2.6 |
| • Southern Africa | ||
| Malawi | 3.9 | 0.3 |
| Zimbabwe | 12.4 | 5.9 |
| Namibia | 3.0 | 1.8 |
| South Africa | 12.4 | 4.9 |
| Swaziland | 15.5 | 4.0 |
High incidences of the tumor had earlier been recorded in a number of sub-Saharan countries, with age-standardized incidence rates of between 19.2 and 28.4 per 100,000 persons/year and with the tumor accounting for about 20% of all malignant disea-ses.4,6,8,23,24 In other reports, the frequency of HCC was expressed only in the latter way: the tumor accounted for 5% of all malignant diseases in Uganda,25 11.2% of those in Ethiopia,26 and 42% of those in Senegal.27 Mozambique, the world’s poorest country in 1986, had the highest recorded incidence of HCC among sub-Saharan countries (101.7 per 100,000 persons/year in males and 31.4 per 100,000 persons/year in females) in an International Agency for Research in Cancer (IARC) registry covering the years 1956 to 1960,28 an incidence 58 times higher than that in United States Caucasians At that time the tumor accounted for 65.5% of all malignant diseases in males and 31% of those in females.29,30
HCC is not uniformly common within sub-Saharan African countries, although the information in this regard is limited by the small number of reliable cancer registries. This phenomenon is most obvious in Mozambique, where the tumor occurs appreciably more often in the eastern regions around Inhamba-ne, Panda, Inharrime, and Morrumbene than in other regions.30 Differences in frequency rates have also been reported between high- and low-lying geographical regions in Swaziland31 and between tribes in Uganda.25 In South Africa HCC is more common in rural than in urban Blacks (ratio 4.4:1.0).32 These differences, at both a sub-continental and a more local level, are in keeping with environmental factors playing an important role, but one which varies in extent geographically, in the etiology of HCC in sub-Saharan Africa.
Changes in incidence of hepatocellular carcinoma in sub-Saharan Black Africans over timeFew studies have been reported on changes over time in the incidence of HCC in sub-Saharan African countries, and the results have been conflicting. Early reports from Uganda showed incidences to be unchanged between 1954–1960 and 1993–1997.33,34 In contrast, the occurrence of the tumor has recently been reported to be increasing in The Gambia.35,36 In a study of changes over time in the incidence of HCC in a large cohort of Black Africans recruited from a number of southern African countries to work on a contract basis in the South African gold mines, McGlashan, et al. recorded a decrease in the incidence of HCC of 32% between 1964 and 1994.37 But there had been, over this time, changes in the relative frequency of recruits from the various countries of origin having different incidences of HCC, and this may have influenced the findings. In particular, the number of recruits from Mozambique, which early on supplied approximately 75% of the mine labor force, decreased significantly over this time.37
Effects of migration on the occurrence of hepatocellular carcinoma in sub-Saharan Black AfricansChronic hepatitis B virus (HBV) infection is the major cause of HCC in sub-Saharan Black Africans.7,9,38 HBV infection resulting in the carrier state in this population occurs to a very large extent in early childhood as a result of horizontal transmission of the virus, and only to a small extent of perinatal infection.39,40 Adult incidences of infection are largely in place by the age of 5 years. Accordingly, when Black Africans migrate to countries in which HBV is not endemic, the carrier rate decreases fairly rapidly, starting in the first generation born in the new environment. This phenomenon, together with the far lesser risk of exposure in the adopted country to another environmental carcinogen, aflatoxin B1, prevalent in sub-Saharan Africa but not in the adopted country, and to dietary iron overload in the African, an additional important cause of HCC in rural Black Africans, account for the appreciably lower incidence of HCC in the generations that follow. The age-standardized incidence of the tumor in African Americans is less than 5 per 100,000 persons/year, although it remains marginally higher than that in Caucasian Americans.41
Age DistributionIn resource-rich countries with low or intermediate incidences of HCC, the patients usually present with the tumor at an age that ranges from the late fifties to the early seventies, with a mean age of approximately 65 years.18,42 Very few patients under the age of 40 years are seen. In contrast, in sub-Saharan Black African patients with HCC there is a distinct shift towards the younger age groups. The mean age of rural-born and -living Black Africans with the tumor in southern Africa is 34.7 years, that of urban-born and -living Blacks 51.0 years, and that of rural-born Blacks who later migrate to the city (usually in early adulthood) 50.9 years.43,44 Nevertheless, the tumor does increase in incidence with increasing age in Black African populations, although in the rural patients it typically decreases in the oldest age groups. With the exception of Mozambique, where men with HCC are significantly younger than women,45 southern African Black men and women with the tumor are of similar ages: for example, the mean age of urban southern African Black men is 50.9 years and that of urban Black women 51.0 years.46
The presence of cirrhosis co-existing with HCC in sub-Saharan Black African patients is not age-related: 59.7% of those less than 30 years of age and 63.8% of those 50 years of age or older have coexisting cirrhosis.47,48 The mean age of rural patients with co-existing cirrhosis is 35.8 years and without cirrhosis 34.3 years.47,48
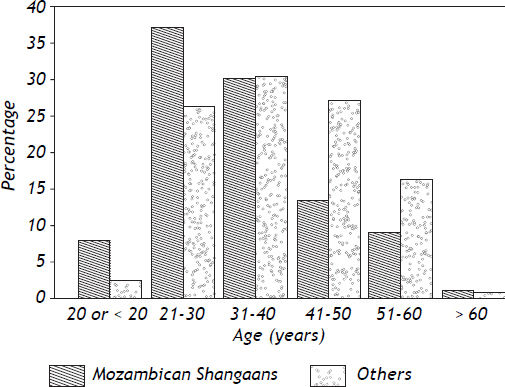
The young age at which the tumor occurs in Black Africans is particularly striking in Mozambique, and more especially in Mozambican men. Prates and Torres reported that 50% of Mozambican Shan-gaans with the tumor were less than 30 years of age when first seen, and the average age of the patients was 33.4 years.45 The incidence of the tumor in males between the ages of 20 and 54 years living in the capital city of Mozambique (Lourenco Marques at that time, now Maputo), was greater than 100 per 100,000 of the population. The young age of Mozam-bican Shangaan males with HCC was later confirmed in two analyses performed in laborers from that country, who presented with the tumor while working on a contract basis in the South African gold mining industry. The miners ranged in age from 18 to 70 years. The age (70 years) above which the miners were not accepted for work in the mines would have influenced the age structure of the mining population studied to a small extent only. In the first analysis, 41% of 328 Mozambican Shan-gaans with HCC were less than 30 years of age, compared with 22.5% of 122 Black miners with the tumor (p < 0.001) who were recruited (with the same exclusion-age and during the same time period) from southern African countries other than Mozambique.49 In the second and later study, the mean age of 271 Mozambican Shangaans with HCC was 33.2 years (range 18 to 60 years) compared with 38.3 years (range 18 to 69 years) in 201 non-Shangaans with the tumor (p < 0.001). A detailed comparison between the age distribution of the Mozambican Shangaan miners with HCC and those from countries other than Mozambique in the second study is shown in figure 1.
With the very high incidence of the tumor in Mozambique and the difference in age distribution between patients in that country and those in developed countries with a low incidence of HCC, it has been estimated that the tumor was approximately 500 times more likely to develop in a Shangaan male aged 25 to 34 years than in a Caucasian male of the same age living in Europe. The difference was only 15 fold in those over the age of 65 years.
The peak incidence of HCC in Black African patients in Uganda was reported to be between 35 and 45 years of age in one analysis25 and between 30 and 50 years (mean age 39.8 years) in a second;50 in Zimbabwe between 31 and 50 years (mean age 32.3 years) in one study51 and 45% of the patients were less than 30 years of age in a second;52 in Nigeria between 20 and 49 years;53 in Kenya between 35 and 45 years in one report54 and 30 and 40 years, with more than 50% of the patients being less than 40 years of age, in a second;55 and in four analyses in Nigeria between 26 and 59 years.56–59 The mean age of the patients in Malawi was 42.7 years,60 in Senegal 38.0 years,61 and in Zaire 35.8 years.62
In patients in a number of sub-Saharan countries, including urban patients in South Africa, the difference in age distribution in comparison with that in low incidence regions of the tumor was less striking. In Ethiopia the peak age incidence was between 41 and 60 years in one study26 and between 31 and 60 years in another,63 in a single study in Zimbabwe the incidence of the tumor continued to rise until the age of 70 years,64 and in one study in Nigeria the age-range was 19 to 86 years with a mean age of 50.6 years.65 The mean age of the patients in The Gambia was recently reported to be 49 years,36 although in an earlier study it was 45 years.66 In a large series of Black civilians in South Africa, of both urban and rural origin, the mean age of the male patients was 51.2 years (range 18 to 78 years; 21.8% less than 40 years of age) and of the female patients 52.1 years (range 18 to 80 years; 23.8% less than 40 years of age).
The presence of cirrhosis co-existing with the tumor does not influence the age of Black African patients, at least not in southern Africa: the mean age of the patients with and without cirrhosis is 35.8 years and 34.3 years, respectively, and cirrhosis is present in 59.7% of those younger than and 63.8% of those older than 30 years of age.67
Black African patients with HBV-induced HCC are, on average, 20 years younger than those with hepatitis C virus-induced tumors.68
Black Africans who migrate in early adulthood from rural areas to the towns or cities in South Africa develop HCC at an average age approximately 20 years older than those who remain in a rural setting.69 This is presumed to be the result of their ‘escaping’ from one or more of the major risk factors for the tumor prevalent in the rural areas (other than HBV, with which they would already have been infected at the time of migration), particularly the hepatocarcinogenic fungal toxin, aflatoxin B1 and, to a lesser extent, dietary iron overload in the African.
The relatively young age at which HCC develops in sub-Saharan Black Africans is also seen in the other high-incidence region of the tumor, namely the Asia-Pacific region. Thus, in Qidong County and the Guanxi Autonomous Region of China, the mean age of the patients is around 40 years70 and in Taiwan most patients are between 41 and 50 years of age.71
HCC is uncommon in South African Caucasians. Those with the tumor have an average age of 55.4 years.72
Gender DistributionIn all populations men have a higher incidence of HCC than do women. Male predominance is even greater in Black African populations. Male:female ratios in these populations range from 1.8:1.0 to 28.0:1.0 in reports from different parts of the subcontinent, with a mean ratio of 3.5:1.0.1,4,13,23,25,26,28,30,33,36,50,52,54,56–60,62,64,66,73–75 The very discrepant sex ratio in one study (28.0:1.0) must have been influenced by a factor or factors other than gender. In contrast, in populations with a low risk of HCC, including South African Caucasians, ratios range from 1.1:1.0 to 3.5:1.0.1,28,33,42,72,76
Male predominance is more striking in young Black Africans.30,75 In one such analysis the sex ratio was 8.1:1.0 in patients less than 30 years of age, compared with 4.2:1.0 in those over 40 years of age.75 By comparison, in populations at low risk of the tumor, male predominance is more evident in older patients, and few patients under the age of 40 years are seen.33,71,76
The increased risk of HCC development in Black African males reflects, in part, their higher rate of chronic HBV infection (approximately twice that in females).38 Moreover, males generally have a greater intake of food (and hence dietary carcinogens, such as aflatoxin B1) than do females. There may also be differences between the two sexes in the rates and efficiency with which ingested chemical carcinogens, such as aflatoxin B1, are metabolized.77 Chronic HBV infection and dietary exposure to afla-toxin B1 are known to have a synergistic hepatocar-cinogenic interaction.78 Moreover, dietary iron overload in the African, another cause of HCC in Black Africans, is more common in men than women because they consume far larger volumes of the iron-rich home-brewed alcohol and they do not menstruate.
Analyses of histocompatibility antigens have not supported a direct genetic basis for the higher incidence of HCC in Black African males than in females.79,80 One study did show that HLA-B2 and HLA-B49 were associated with an increased risk of tumor formation in this population, but no distinction was made between the sexes.81
Hepatocellular Carcinoma in Black African ChildrenHCC occurs in Black African children, although with a far lower incidence than in adults.82–85 The usual age at presentation is between five and 15 years and the male:female ratio is 2.0–3.0:1.0. The symptoms and clinical signs of the tumor in children do not differ significantly from those in adults. Chronic HBV infection is almost invariably the cause of the malignant transformation in Black African children.84 In contrast, the tumor is rare in children in resource-rich countries, and when it does occur the children almost always suffer from one of the rare inherited diseases known to be complicated by this tumor, such as α1 antitrypsin deficiency, hereditary tyrosinemia, glycogen storage disease (type 1), or ataxia telangiectasia.
Although the fibrolamellar variant of hepatocellular carcinoma occurs very rarely in sub-Saharan Black adults, it is seen in Black children, in whom it accounts for approximately 9% of HCCs.86