Hepatitis B virus (HBV) is a global public health problem, with an estimated 296 million chronic carriers worldwide in 2019, causing 820,000 annual deaths from complications such as hepatocellular carcinoma (HCC) and cirrhosis [1]. Current potent anti-HBV agents (nucleos/tide analogues [NA]) effectively suppress viral replication and improve long-term survival. However, hepatitis B surface antigen (HBsAg) loss (i.e., a functional cure) is rarely achieved [2,3].
Canadian patients with chronic hepatitis B are predominantly of Asian background [4], prior study showed ∼53% have HBV genotype B and C, although all major HBV genotypes were found [4]. Despite universal childhood vaccination in Canada since the 1990s, CHB remains a public health challenge due to significant immigration, especially from HBV-endemic regions [5]. Action Hepatitis Canada [6] highlights inadequate progress in HBV elimination in Canadian provinces, emphasizing the urgent need for updated prevalence estimates to inform policies that support equitable care access. A prevalence assessment by the Canadian Liver Foundation in 2013 projected 272,640-467,222 HBV-infected individuals in Canada in 2020, predominantly new immigrants from HBV-endemic regions [5]. While HBV infection is a reportable disease in Canada, reporting practices remain inconsistent across health jurisdictions. In 2019, there were 3,790 reported cases of CHB in Canada (10.2/100,000 population) [7]. The Ontario Burden of Infectious Disease Study showed hepatitis B as the fifth leading cause of morbidity and mortality [8]. Despite effective anti-HBV therapies, recent data suggest excess mortality among CHB patients [9,10]. Moreover, there is limited data on the survival of CHB patients in North America.
We, therefore, aimed to determine the incidence and prevalence of CHB in Alberta (population 4.4 million), Canada, between 2012 and 2021, using a well-defined laboratory-based algorithm. We also evaluated overall survival and liver transplant-free survival in this population and described survival rates according to demographic variables.
2Patients and methods2.1Study design and data sourcesDetails about Alberta Health Services are provided in Appendix 1.
Using a population-based retrospective cohort design, we searched Alberta Analytics administrative databases including the Alberta Provincial Laboratory database, between April 1, 2012, and March 31, 2021 (fiscal years [FYs] 2012 through 2020) to identify patients with CHB. We further searched three administrative databases from Alberta Analytics and Alberta Health, including the National Ambulatory Care Reporting System (NACRS), Discharge Abstract Database (DAD), and Physician Claims to extract liver transplantation and comorbid HCC data [11].
Appendix 2 contains codes for extracting liver transplantation and HCC data.
We obtained demographics, including the cohort's date of birth and sex, and cause of death from Alberta Vital Statistics. We further searched Pharmaceutical Information Network (PIN) to extract Drug Identification Number (DIN) for the antiviral therapy recommended for use in patients with CHB [11] (Appendix 3).
2.2Study populationA laboratory-confirmed CHB case was defined as12: 1) detection of HBsAg or hepatitis B e antigen (HBeAg) for at least six months; or 2) immunoglobulin M antibody to Hepatitis B core antigen (anti-HBc IgM) negative and at least one of the following: HBsAg positive or HBeAg positive (Fig. 1).
Flow chart illustrating data sources, case definitions and exclusion criteria to identify laboratory-confirmed incident and prevalent cases of chronic hepatitis B in Alberta between fiscal years 2012-2020.
Abbreviations: AHCIP, Alberta Health Care Insurance Plan; anti-HBc IgM, Immunoglobulin M antibody to hepatitis B core antigen; CHB, Chronic hepatitis B; FYs, Fiscal years; HBeAg, Hepatitis B e antigen; HBsAg, Hepatitis B surface antigen.
Individuals were excluded if: 1) they were non-residents of Alberta; 2) non-registrants with the Alberta Health Care Insurance Plan (AHCIP); or 3) their death was recorded in Alberta Vital Statistics during a FY of interest. Furthermore, using linked laboratory and administrative databases, individuals who underwent liver transplantation ≤ 90 days of laboratory confirmation of CHB (N=12) were excluded from the incident cohort (i.e., to avoid counting them in the analysis of the natural history of CHB).
2.3Study outcomesThe overall incidence and annual incidence of CHB for each fiscal year of our study period were determined in Alberta (FYs 2015-2020). We used a washout period of 3 years (FYs 2012-2014) to avoid including prevalent cases. The point prevalence of CHB in Alberta for each FY (2015 through 2020) was determined. We further calculated age/sex-adjusted incidence and prevalence (i.e., using the direct standardization method with the 2016 Canadian population as reference [12]). In calculating incidence and prevalence, the midyear population of Alberta was considered at risk [12], and the 2016 Canadian population was used as a reference to calculate age/sex-adjusted estimates [12].
To describe the natural history of CHB in Alberta, the main outcomes were 1- and 5-year overall survival. Liver transplant-free survival for the respective periods were secondary outcomes. Date of the laboratory confirmation was considered the date of diagnosis, and all subjects were followed up from diagnosis (index date) until death, liver transplantation or censoring (i.e., censoring occurred upon deregistration from the AHCIP due to migration out of the province or March 31, 2021, whichever came first). Survival rates were compared with the expected survival of the age/sex-matched 2016 Canadian population using the standardized mortality ratio (SMR).
2.4Age- and sex-stratified analysis and exposure variablesIncidence and prevalence were stratified by sex and age at diagnosis and grouped into the following categories: 0-17, 18-44, 45-64 and ≥65 years. We further assessed CHB incidence and prevalence among children <5 years, a crucial target population for global hepatitis elimination strategy (Appendix 4).
Analysis of incidence and prevalence of CHB among the study population was stratified based on demographic variables (sex and age at diagnosis). We also explored the survival rates stratified by sex and age at diagnosis, and examined the impact of age, sex, year of diagnosis, comorbid HCC and receiving anti-HBV treatment on survival of patients.
2.5Data analysisDescriptive statistical methods were used to report the characteristics of the cohort (i.e., continuous variables were summarized as the median and interquartile range (IQR), and categorical variables were expressed as number and percentile). Incidence and prevalence were expressed per 100,000 persons, along with their corresponding 95% confidence intervals (CI). Trends of annual incidence and point prevalence were analyzed using a Poisson regression model. Incidence and prevalence rate ratios and their 95% CIs were calculated to assess sex and age differences in the incidence and prevalence (i.e., using males and age group 0-17 years as the references). Incidence and prevalence were considered significantly different if the rate ratio CIs did not include one. Survival of the incident cohort was examined using Kaplan-Meier survival analyses with comparisons across sex and age groups using the log-rank test. The impact of sex, age, year of diagnosis, comorbid HCC and receiving anti-HBV treatment on the survival of patients was examined using a Cox proportional hazards model with time-varying covariates (i.e., considering comorbid HCC and receiving anti-HBV treatment as time-varying covariates). The assumption of proportional hazards was examined and confirmed to be met in our analysis. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 16.1 (Stata-Corp, College Station, Texas, USA) and Microsoft Excel (version 16.16.27; Microsoft Corporation, Redmond, WA).
2.6Ethical statementThis study received ethics approval from the University of Calgary Conjoint Health Research Ethics Board (Ethics approval ID: REB18-1493), according to the Declaration of Helsinki.
3Results3.1Development of the study cohortA total of 14,951 individuals tested positive for HBsAg in Alberta between April 1, 2012, and March 31, 2021. A total of 26,963 individuals tested negative for anti-HBc IgM, and 1,219 individuals tested positive for HBeAg during the study period.
The study cohort was classified into the following groups according to the confirmatory laboratory criteria for CHB infection: 1) two positive test results for HBsAg at least six months apart (n=7,416, 57.4%), 2) a positive test result for HBsAg followed by a negative test result for anti-HBc IgM (n=4,435, 34.4%), 3) two positive test results for HBeAg at least six months apart (n=719, 5.6%), and 4) positive test result for HBeAg followed by a negative test result for anti-HBc IgM (n=353, 2.8%). For those meeting more than one confirmatory laboratory criterion for CHB, the earliest recorded criterion was considered as index. Ultimately, 9,557 individuals with laboratory-confirmed CHB infection in Alberta during the study period were identified, of which 21 were non-registrant with the AHCIP (Fig. 1).
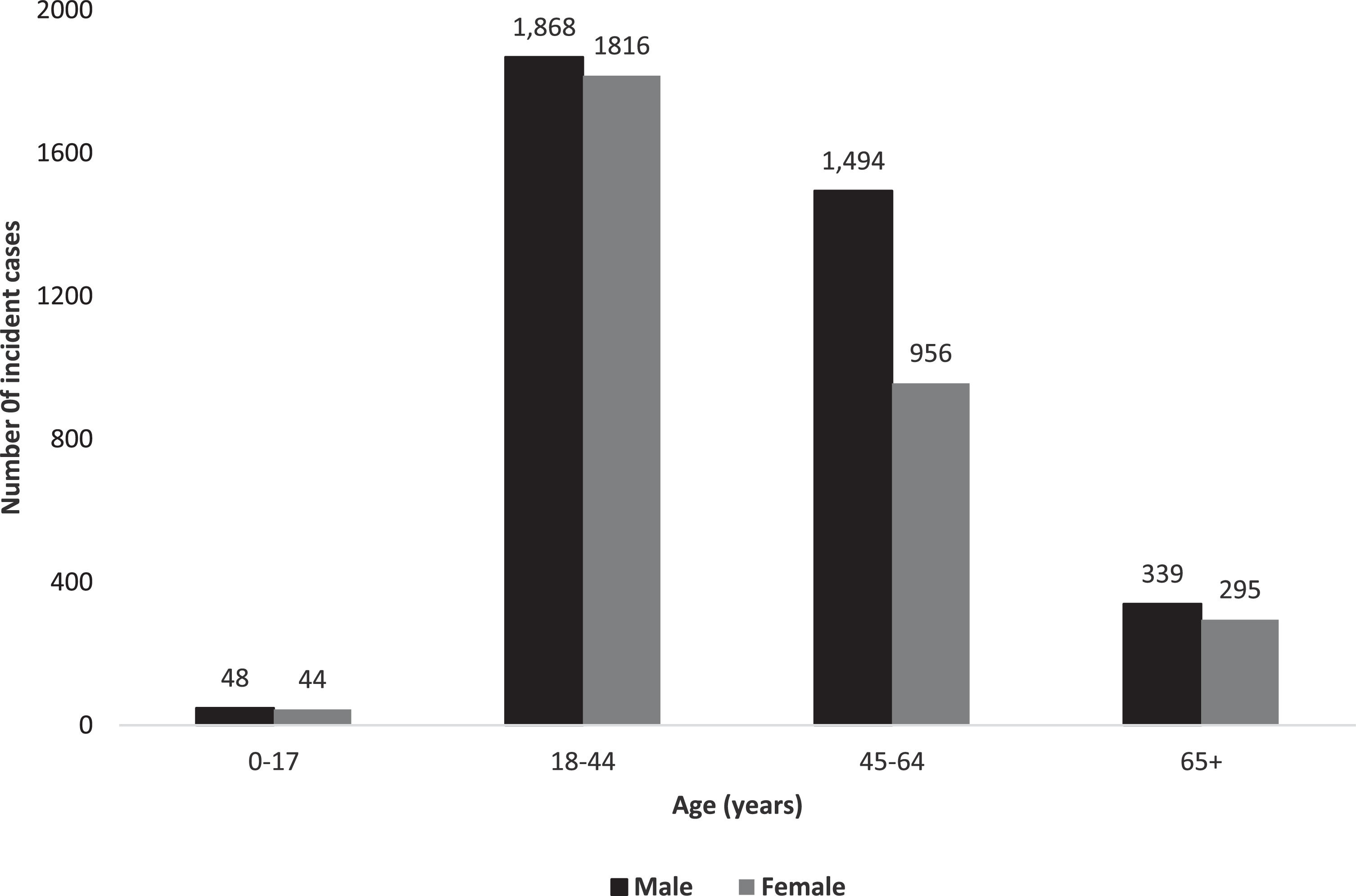
3.2Characteristics of our study cohortOverall, 9,536 prevalent individuals with CHB resided in Alberta at some point between April 1, 2012, and March 31, 2021. We identified 6,860 cases with incident diagnosis of CHB between 2015-2020 in Alberta (Fig. 1). Frequencies of incident CHB cases by sex and age are illustrated in Fig. 2. The majority (54.7%) were males, and median age at diagnosis was 42.0 years (IQR 34.0-54.0 years). 8,810 (92.4%) prevalent cases and 6,273 (91.5%) incident cases were born before 1990; median age at diagnosis was 44 years (IQR 36-55 years).
Of the 6,860 incident cases of CHB in Alberta between 2015-2020, 5,869 (85.6%) patients did not undergo HBsAg testing during the washout period (April 1, 2012, to March 31, 2015). Among the remaining 991 (14.5%) patients who underwent HBsAg testing during the washout period, 61 (6.2%) patients tested negative, 920 (92.9%) patients tested positive but did not meet the confirmatory laboratory criteria for CHB. Ten (1.1%) patients had fluctuating HBsAg test results, (i.e., test alternated between positive and negative), likely indicating low-level seropositivity at threshold of assay detection limits.
3.3Incidence, prevalence, and temporal trendsThe overall age/sex-adjusted incidence of CHB between 2015-2020 was 27.1 per 100,000 person/years (95% CI 26.5-27.6). During the study period, the age/sex-adjusted incidence of CHB decreased from 36.4 per 100,000 (95% CI 35.8-37.0) in 2015 to 13.4 per 100,000 (95% CI 13.0-13.8) in 2020 (Fig. 3 and Appendix Table 1). The Poisson regression model revealed a statistically significant decrease in the annual incidence of CHB from 2015 to 2020 (IRR 0.847 [95% CI 0.843-0.851], p<0.001).
Age/sex-adjusted period prevalence of CHB in 2020 was 210.3 per 100,000 (95% CI 208.8-211.8). The age/sex-adjusted point prevalence of CHB increased from 98.9 per 100,000 (95% CI 97.9-100.0) in 2015 to 210.3 per 100,000 (95% CI 208.8-211.8) in 2020 (Fig. 4 and Appendix Table 2). The Poisson regression model revealed a statistically significant increase in the point prevalence of CHB from 2015 to 2020 (IRR 1.148 [95% CI 1.147-1.151], p<0.001).
3.4Differences in incidence and prevalence based on age and sexAge-adjusted incidence was lower among females compared to males (24.5 per 100,000 person/years [95% CI 23.8-25.3] vs 29.6 per 100,000 person/years [95% CI 28.8-30.4]; incidence rate ratio (IRR) 0.83 [95% CI 0.80-0.87]). The incidence of CHB also highly depended on age. The highest sex-adjusted incidence occurred among individuals aged 45-64 years (37.6 per 100,000 person/years [95% CI 36.4-38.8] versus 1.7 per 100,000 person/years (95% CI 1.4-2.0) in <18-year-old category; IRR 23.4 [95% CI 19.4-28.4]) (Table 1). This age pattern was also observed among males, with the highest incidence occurring among males aged 45-64 years (45.4 per 100,000 person/years [95% CI 43.6-47.3]). In comparison, females aged 18-44 years had the highest incidence at 36.5 per 100,000 person/years (95% CI 35.0-38.0).
Overall incidence (Fiscal years 2015-2020) and point prevalence (Fiscal year 2020) of chronic hepatitis B in Alberta.
CI, confidence interval.
Age-adjusted period prevalence in 2020 was higher among males compared to females (226.7 per 100,000 [95% CI 224.5-228.9] vs 194.1 [95% CI 192.1-196.1]; rate ratio 0.86 [95% CI 0.85-0.87]). The highest adjusted period prevalence was observed among the age group 45-64 years (332.7 per 100,000 [95% CI 329.2-336.3] versus 7.2 per 100,000 [95% CI 6.6-7.9] in the <18-year-old category; rate ratio 46.3 [95% CI 42.5-50.1]) (Table 1).
3.5Clinical characteristics of the incident cohortThe 6,860 incident cases of CHB were followed for a total of 23,107 person-years from diagnosis. During a median follow-up of 3.6 years (IQR 2.0-4.9 years), 12 (0.18%) patients underwent liver transplantation, 249 patients (3.7%) were diagnosed with HCC, and 142 (2.1%) patients died. No patient died after liver transplantation. Only 1,262 patients (18.4%) received HBV treatment. Between FYs 2015-2020, the annual mortality rate was 0.56 per 100,000 (95% CI 0.47-0.66), with males showing a higher annual mortality rate compared to females (i.e., an annual mortality rate of 0.75 per 100,000 in males versus 0.37 per 100,000 in females, p<0.0001). Furthermore, individuals aged ≥65 years had a significantly higher annual mortality rate compared to other age groups, with rates of 1.8 per 100,000 among those aged ≥65 years and 0, 0.18, and 1.1 per 100,000 in the 0-17, 18-44, and 45-64 age groups, respectively (Appendix Table 3). The cause of death, available in 93% (132/142) of patients, was liver-related in 44 (33.4%). Further details on cause of death are provided in Appendix 5.
Among the incident CHB cohort, 1,262 (18.4%) patients had at least one prescription dispensed from community pharmacies for anti-HBV medication. In total, 24,736 prescriptions were dispensed for anti-HBV therapy for this cohort between 2015-2020. Details about the types of anti-HBV treatment are outlined in Appendix 3.
3.6Survival analysis and description of survival according to age and sexOverall survival of 6,860 incident cases of CHB during a median follow-up of 3.6 years (IQR 2.0-4.9 years) from diagnosis is illustrated in (Appendix Fig. 1). The estimated 1- and 5-year survival rates were 99.2% (95% CI 98.9%-99.4%) and 97.2% (96.7%-97.7%), respectively, corresponding to an SMR of 3.9 (95% CI 3.3-4.6) compared with the general Canadian population.
Survival was lower among men compared with women (p=0.001) (Fig. 5-A). In women, estimated 1- and 5-year survival rates were 99.5% (95% CI 99.2-99.7%) and 98.1% (97.5-98.6%), respectively, corresponding to an SMR of 3.2 (95% CI 2.4-4.2). In men with CHB, estimated 1- and 5-year survival rates were 98.9% (95% CI 98.5-99.2%) and 96.4 (95% CI 95.5-97.2%), respectively, corresponding to an SMR of 4.2 (95% CI 3.4-5.2). In addition to sex, lower survival was associated with an older age at diagnosis (p<0.001) (Fig. 5-B).
We conducted further survival comparisons within our incident cohort, categorizing it based on both sex and age groups (i.e., males aged 0-17, 18-44, 45-64, and ≥65 years, and females aged 0-17, 18-44, 45-64, and ≥65 years). This analysis showed the lowest survival probability among males aged ≥65 years, with 1-year and 5-year survival probabilities of 95.6% [92.7%-97.4%] and 83.2% [76.0%-88.3%], respectively, followed by females aged ≥65 years, with 1-year and 5-year survival probabilities of 97.2% [94.5%-98.6%] and 90.0% [84.8%-93.5%], respectively (Appendix Table 4 and Appendix Fig. 2).
In both univariate and multivariate Cox proportional hazards analysis with time-varying covariates, males (hazard ratio [HR] 1.7; 95% CI 1.2-2.5) and older patients at diagnosis (HR, 1.08; 95% CI 1.07-1.09) had a higher risk of mortality. However, comorbid HCC (HR, 0.9; 95% CI 0.7-1.2), receiving anti-HBV treatment (HR, 1.2; 95% CI 0.9-1.5) and year of diagnosis (HR, 1.1; 95% CI 0.9-1.2) were not significantly associated with mortality (Appendix Table 5).
In the incident cohort of CHB, the 1- and 5-year transplant-free survival rates of CHB patients after diagnosis was similar to that of overall survival.
4DiscussionThis population-based study represents an up-to-date estimate of the trends in the epidemiology and natural history of CHB in a large Canadian population. The major strength of our study is its population-based approach in a low-endemic region which supports the generalizability of our findings to similar areas, especially within North America and Western Europe. In contrast, existing studies on epidemiology and the natural history of viral hepatitis are mostly restricted to selected populations [10,13,14] or conducted in high-endemic countries [15]. Our study is also strengthened by the case ascertainment utilizing the Provincial Laboratory database to identify all individuals with laboratory-confirmed CHB in the Alberta population. To our knowledge, this is the largest population-based study evaluating the natural history of CHB in a high-income country with low endemicity of HBV followed beyond 2016.
Our study updates the North American data regarding the epidemiology of CHB [7,16–20]. Recent epidemiologic reports of CHB from low-endemic regions, i.e., 21 European countries [19], and the USA[20] indicated incidence between 2.6 and 5 cases per 100,000 in 2020, respectively. The Public Health Agency of Canada (PHAC) reported incidence of 10.2 and 11.6 per 100,000 in 2019 for Canada and Alberta, respectively [7]. The annual incidence and point prevalence in our study of 13.4 and 210.3 per 100,000, respectively, are among the highest reported in low-endemic regions. This is likely due to our study's case ascertainment and implementing a laboratory-based hepatitis B serology algorithm rather than ICD codes or chart review. Other North American studies on the epidemiology of CHB mainly provide data on the point prevalence of cases rather than comprehensive assessment including trends of both incidence and prevalence cases. For example, Binka et al. [18] identified 44,483 cases of CHB in British Columbia (BC) using data from the BC Hepatitis Testers Cohort between 1990-2015. A recent systematic review [17] estimated 1.47 million foreign-born and 1.89 million total persons (Foreign- and U.S.A.-born) living with CHB in the USA in 2018. The up-to-date estimates of incidence and prevalence in our study are essential for investigating distributions and determinants of CHB.
Between 2015 and 2020, the incidence of CHB in Alberta declined by almost 64%, which is consistent with other studies conducted in the HBV low-endemic regions. Data from Canada [7] and 16 low-endemic European countries [19] revealed an overall decline in incidence of CHB from 12.3 per 100,000 in 2010 to 10.2 in 20197 and from 6.6 per 100,000 in 2015 to 2.8 in 202020, respectively. In contrast to declining incidence of CHB in our region, the prevalence almost doubled during the same study period. This upward trend in CHB prevalence aligns with other studies confirming increasing prevalence of CHB in North America [5,17,21]. A modeling study conducted in 2013 suggested an increase in the number of prevalent cases of CHB by 23,000-30,000 cases between 2006 and 2020 in Canada [5]. Similarly, a systematic review restricted to foreign-born individuals living in the USA suggested that the prevalence of CHB increased from 1.32 million in 2009 to 1.47 million in 201818,22.
The declining incidence of CHB in all age groups in Alberta is most likely related to the implementation of routine HBV immunization and improved screening strategies [7]. However, interrupted routine health care services following the COVID-19 pandemic may contribute to the apparent decline in estimation of the incidence of CHB in 2020 in Alberta [22]. For instance, a recent study from Public Health Ontario reported significant decreases in HBsAg testing (i.e., 15-33%) and HBV DNA testing (i.e., 20-37%) during the COVID-19 pandemic compared to the corresponding months in 201924. It is worth noting that between July 2020 and June 2021, Alberta had the lowest number of international immigrants in 13 years due to COVID-19 travel restrictions [23,24]. The phenomenon of "compounding prevalence" can explain the rise in prevalence of CHB, despite a decrease in incidence. Compounding prevalence has been observed in other chronic, incurable diseases (i.e., inflammatory bowel disease), where there are more patients with the newly diagnosed disease than those who have died from it [25]. Moreover, the development of safe and potent anti-HBV oral therapy (NA) has improved CHB prognosis in those who are able to access treatment [2,3,26–29].
The higher CHB risk in males (male/female ratio of 1.2) aligns with ratios of 1.2-1.3 in other studies [7,18,19], possibly linked to overall elevated risk of chronic liver disease among males both due to biological (sex hormone differences) as well as lifestyle (e.g., higher prevalence of alcohol-related liver disease [30]). The burden of CHB was also age-dependent, and the highest disease incidence and prevalence were observed among the middle-aged group (individuals aged 45-64 years), consistent with data by the PHAC reporting the highest incidence among individuals aged 40-59 years [7]. The most likely factors contributing to higher CHB incidence and prevalence among the age groups 45-64 years followed by 18-44 years included the universal childhood vaccination programs in Canada and catch-up vaccination program in older individuals, born or lived in an HBV endemic region that lacked universal infant vaccination programs, and to a lesser degree, injecting drug use or risky sexual behaviour.
In the current study, we analyzed survival for the incident cohort of CHB patients of all age groups and presumed racially diverse [4] (i.e., due to the retrospective nature of our study and its reliance on administrative databases, it was not feasible to evaluate ethnicity within our study). We also did not restrict the analysis to treatment status, comorbidities, and CHB outcomes. The estimated 1- and 5-year survival rates were 99.2 and 97.2%, respectively. These figures align with other studies confirming premature death among CHB patients when evaluating 5 and 10-year survival rather than overall survival [13,14]. A multicentre European reported a 5-year survival rate of 95.9% among Caucasian patients ≥16 years with no history of HCC and HCV/HIV/HDV coinfections who received NA for at least one year (median 6 years) at entry [14]. HCC (diagnosed in 6.0% of incident cohort) had been associated with overall mortality [14]. In the Japanese cohort of CHB patients with no history of HCC, HCV coinfection and other causes of chronic liver disease, 5-year and 10-year survival rates did not differ among NA-treated and untreated patients [13]. However, overall survival differed according to treatment status [13]. Similarly, our observation that CHB treatment status and HCC did not affect 5 and 10-year survival does not preclude their potential impact on overall survival as our median follow-up was shorter (3.6 years) and we had a relatively small percentage of our cohort developing HCC (3.7%).
Our results are in keeping with other reports suggesting excess mortality among CHB patients compared with the general population [10,31,32] or uninfected cohort [9]. All-cause SMR for our incident cohort (i.e., SMR of 3.2) is among the highest reported by studies conducted in high-income countries with low endemicity of CHB [10,14,32]. The SMR difference between our cohort and others is primarily due to varying study populations (recruitment sources and entry criteria). Studies in the USA [10] and France [31] recruited patients from the Chronic Hepatitis Cohort Study [10] and excluded HIV/HCV coinfections [31], respectively, potentially leading to improved outcomes and lower SMRs of 1.85 [10] and 1.7 [31]. Furthermore, a European multicenter study [14] on NA-treated patients without HCC and HCV/HIV/HDV coinfections in liver clinics reported no difference in mortality among CHB patients and the general population (SMR of 0.82).
Studies from the USA [33] and Canada [34–36] highlighted suboptimal evaluation and management for CHB patients, which explains the persistent excess mortality in the era of available effective HBV therapeutics.
We did not observe a significant difference in survival according to HCC comorbidity, treatment status and year of diagnosis. In keeping with other studies, we identified two independent predictors of mortality: older age at diagnosis and male sex [10,31], which may relate to accelerated disease progression among older patients [37,38], the apparent general association of older age with a higher risk of death from any cause, and higher prevalence of metabolic dysfunction associated steatotic liver disease (formerly called non-alcoholic fatty liver disease [30]) and alcohol use [39] among males.
Some limitations of our study should be acknowledged. Most importantly, we may underestimate the burden of CHB in Alberta because the data presented in the current study included only those tested for and diagnosed with CHB. A Canadian study found immigrants received CHB diagnoses about six years post-migration [36]. despite a 3-year washout period for laboratory confirmation, misclassification may occur, overestimating CHB incidence. No validated washout period exists to distinguish incidents from prevalent cases. Determining the optimal washout period through sensitivity analysis was not feasible due to retrospective laboratory data, some of which were inaccessible before 2012. Notably, 85.6% of incident cases in our study lacked HBsAg testing during the washout period. Unfortunately, immigration status is not available in Alberta health related databases including the provincial laboratory database. Therefore, we were not able to evaluate our study outcomes based on immigration status. Finally, our natural history study's limitation includes a relatively short 3.6-year follow-up, contrasting with longer periods (5.4-8.1 years) in comparable studies [9,10,14,31,32]. Considering that many people with CHB are asymptomatic, extended observation is essential to comprehensively understand the disease's natural history.
5ConclusionsOur findings fill the knowledge gap regarding the HBV infection epidemiology in Canada, aiding surveillance and planning strategies to decrease the disease burden at the public health levels. The incidence of 27.1/100,000 person/years between 2015-2020 is among the highest reported in high-income countries with low HBV endemicity. The incidence of CHB in our region declined by almost 64%, while the point prevalence nearly doubled between 2015 and 2020. Despite improved anti-HBV therapies, CHB patients had four times higher all-cause mortality compared to general population. Thus, eliminating barriers to the recommended HBV care, from screening to medication access, is crucial in fulfilling Canada's commitment to achieving the WHO 2030 goals for viral hepatitis elimination.
This work was funded in part through an investigator-sponsored research grant by Gilead Sciences. GSK was funded by a studentship from the Canadian Liver Foundation.