The prevalence of nonalcoholic fatty liver disease (NAFLD) is continuing to rise in many countries, paralleling the epidemic of obesity worldwide. In the last years, the concept of metabolically healthy obesity [MHO, generally defined as obesity without metabolic syndrome (MetS)] has raised considerable scientific interest. MHO is a complex phenotype with risks intermediate between metabolically healthy individuals with normal-weight (NWMH) and patients who are obese and metabolically unhealthy (MUO, i.e. obesity with MetS). In this review we aimed to examine the association and pathophysiological link of NAFLD with MHO and MUO. Compared to NWMH individuals, patients with obesity, regardless of the presence of MetS features, are at higher risk of all-cause mortality and cardiovascular events. Moreover, MHO patients have a greater risk of NAFLD development and progression compared to NWMH individuals. However, this risk is generally lower than that of MUO patients, suggesting a stronger adverse effect of coexisting MetS disorders than obesity per se on the severity of NAFLD. Nevertheless, since MHO is a dynamic state (with a significant proportion of MHO subjects progressing to MUO over time) and NAFLD itself may predict the transition from MHO to MUO, we believe that any effort should be made to identify NAFLD in all obese individuals, although they appear to be “metabolically healthy”. Future research is needed to better understand the role of NAFLD and other pathogenic factors potentially involved in the transition from MHO to MUO and to elucidate how this transition may affect the presence and severity of NAFLD.
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of exceedingly common liver conditions [ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) which may progress to cirrhosis and hepatocellular carcinoma (HCC)], observed in individuals without competing causes of chronic liver disease [1]. In clinical practice, NAFLD is often diagnosed and staged non-invasively with imaging techniques and laboratory surrogate biomarkers [2].
Further to the aforementioned spectrum of liver disease, NAFLD also entails systemic derangements of multiple organ systems, the best characterized of which are the coexistence of cardiovascular disease, certain extra-hepatic cancers (mainly colorectal cancers), type 2 diabetes mellitus (T2DM) and other features of the metabolic syndrome (MetS) [3]. However, the link between NAFLD and MetS is more complex than previously thought. Indeed, the MetS shows mutual and bi-directional associations with NAFLD, such that NAFLD is now considered to be both a cause and a consequence of the MetS [3–6]. Nevertheless, NAFLD may also result from some specific genetic polymorphisms [e.g. the patatin-like phospholipase domain-containing protein 3 (PNPLA3) I148M variant and the trans-membrane 6 superfamily member 2 (TM6SF2) E167K variant] [7], but it reasonable to hypothesize that these forms of “genetics-related NAFLD” are not entirely superimposable to the “MetS-related NAFLD” forms from a biological and clinical point of view [8].
Owing to epidemiological and clinical reasons, NAFLD has become a public health problem [9] that affects nearly a quarter of the world's adult population and poses a considerable economic burden on the healthcare systems of many developed and developing countries [9].
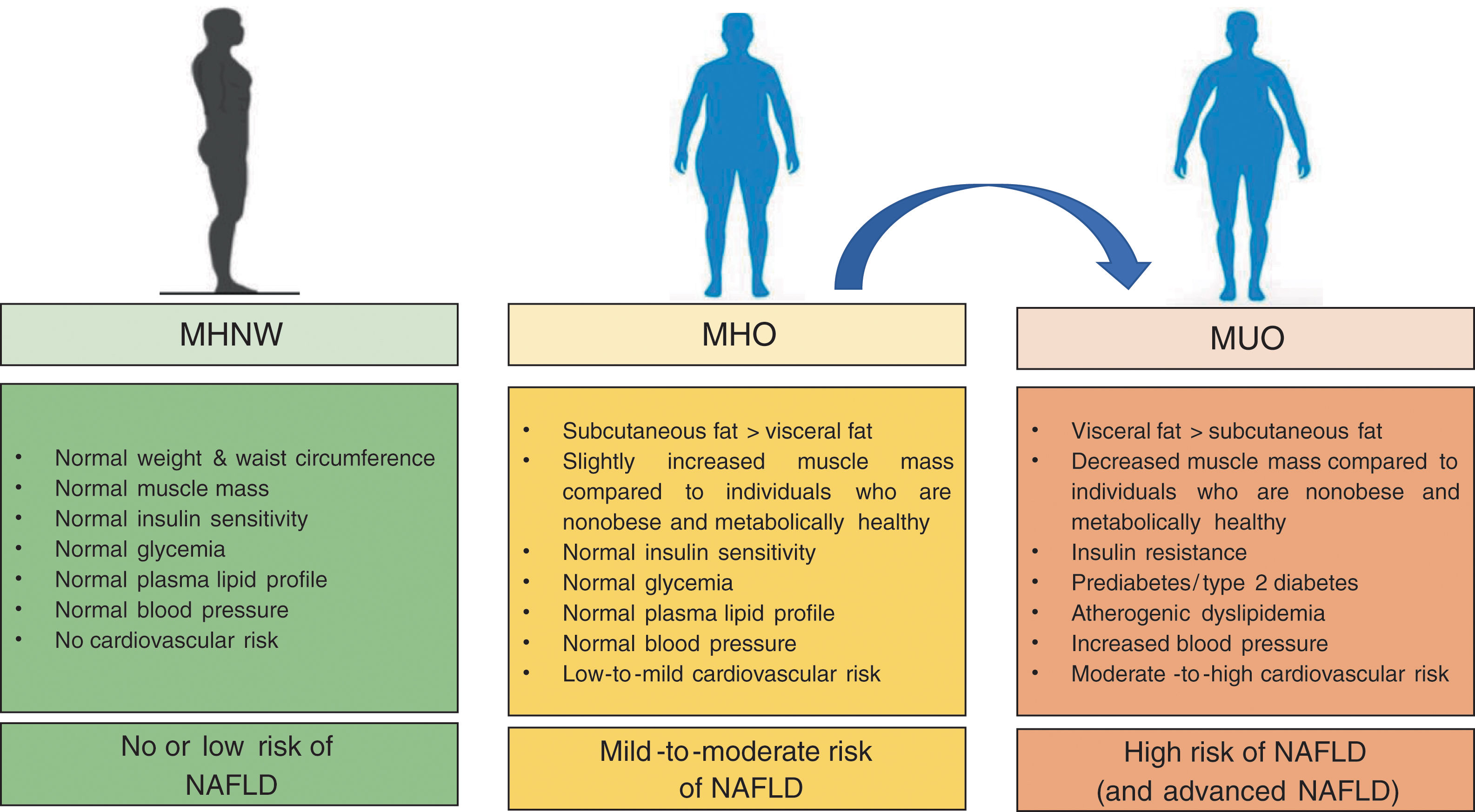
1.2Obesity definition, burden and outcomesObesity, which is a public health problem in many areas of the world [10], is defined as a body mass index (BMI) ≥30kg/m2 in Caucasian populations, or BMI ≥25kg/m2 in Asian-Pacific populations [11]. Usually, the major clinical outcomes of obese individuals are excess risk of developing MetS features, cardiovascular disease and several cancer types which, collectively, lead to premature mortality, thus justifying the concept of “metabolically unhealthy obesity” (MUO) [12]. Conversely, a subset of obese individuals appears to be spared from the MetS features and these obese individuals are, therefore, defined “metabolically healthy”, although there is no universally accepted definition of “metabolically healthy obesity” (MHO) [13]. Other individuals, despite their BMIs are normal, have an increased intra-abdominal accumulation of body fat and other coexisting MetS features [14]. These individuals are “metabolically unhealthy normal weight” (MUNW), have increased mortality rates, and therefore are non-obese but not metabolically healthy [15,16]. Attesting to the importance of intra-abdominal body fat distribution, the diagnostic criteria for MetS include (ethnicity-specific) measures of waist circumference rather than BMI [17].
1.3Aim of the reviewGiven the epidemic prevalence of NAFLD [9] and obesity [18] worldwide, and the pathophysiological link between these two conditions [19], in this review article we have briefly examined the inter-relationships and the underlying mechanisms linking MUO and MHO to risk of NAFLD. To this end, we have extensively searched the PubMed database for articles that have been published between 1990 and 2019 (using the following key-words: obesity; abdominal obesity; MHO; MUO; BMI, waist circumference; AND epidemiology OR pathophysiology). Retrieved articles were selected based on the authors’ agreement and only those publications unanimously judged relevant were retained.
2EpidemiologyIn the last decade, a number of studies have investigated the inter-relationships of MUO and MHO with NAFLD.
2.1Association between MUO and NAFLDIn severely obese patients with coexisting MetS features the prevalence of imaging-defined NAFLD is approximately 90–95% and more than one third of these patients have NASH on histology [20]. Indeed, increased values of BMI and waist circumference are not only associated with the presence of NAFLD [21], but also with an increased risk of liver disease progression, especially in older patients [22,23]. This may partly due to the fact that NAFLD is more strongly associated with visceral fat than subcutaneous fat [24–27]. Compared to subcutaneous fat, visceral adipose tissue is associated with higher lipolytic rate, greater insulin resistance [20,24–27] and increased release of several pro-inflammatory and pro-fibrogenic mediators, which may promote the development and progression of NAFLD [20,28]. All these mechanisms account for the finding that expanded and inflamed (dysfunctional) visceral adipose tissue is one of the strongest risk factors for developing the more advanced forms of NAFLD [20,28–30].
The coexistence of MetS features is also extremely common in patients with the so-called “cryptogenic” cirrhosis, thereby suggesting that most cases of cryptogenic cirrhosis may be burnt-out NASH [31,32]. Coexisting metabolic co-morbidities of MUO, principally T2DM, also contribute to the development and progression of NAFLD and vice versa [29,33,34].
Recently, an ever-increasing number of observational studies have estimated the prevalence of concurrent MUO and NAFLD in Asian individuals (as extensively reviewed in [35]). This is relevant because Asian individuals tend to have more visceral adipose tissue than other ethnic groups [35], and they also are more susceptible to developing NAFLD at a lower BMI than Caucasian individuals [20,35].
2.2Association between MHO and NAFLDThe MHO is usually defined by the presence of the following pathophysiological features: (a) reduced accumulation of intra-abdominal visceral fat and ectopic fat for any given level of total adiposity, (b) preserved insulin sensitivity, and (c) lower degree of systemic and adipose tissue inflammation when compared to obese patients with coexisting MetS features [36]. The MHO phenotype is relatively common in clinical practice, occurring in up to one-third of obese individuals [36]. However, there is no universally accepted definition of MHO and the existence of this condition remains controversial [13]. This is likely due also to the fact that MHO is a dynamic state with a significant proportion of MHO subjects who will progress to MUO over time and that MHO individuals, even in the absence of coexisting MetS, have an increased risk of all-cause mortality and cardio-metabolic events compared to non-obese individuals [37].
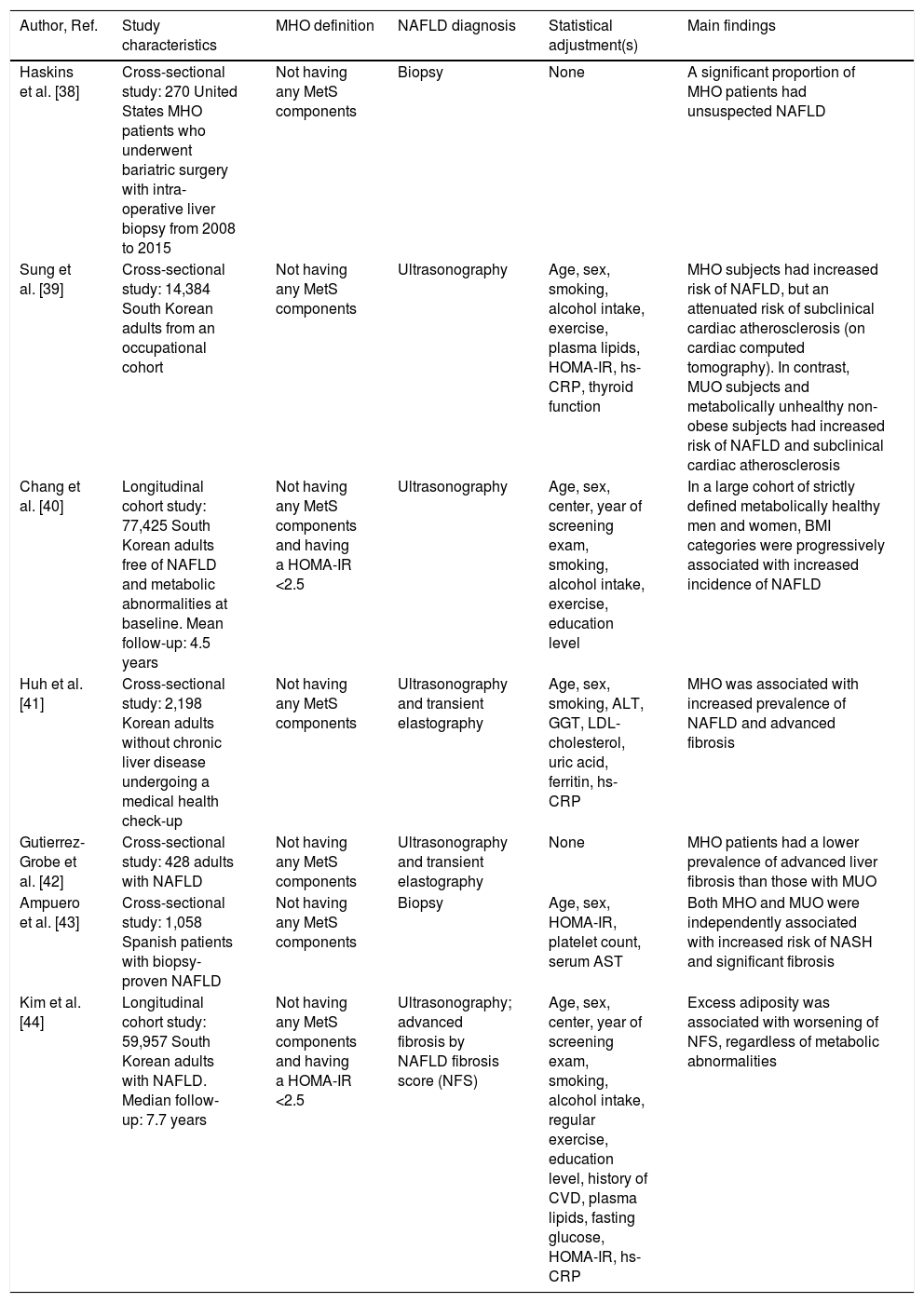
Presently, little is known regarding the impact of MHO on the risk of NAFLD. However, emerging epidemiological evidence indicates that MHO may significantly be associated with a greater risk of having or developing NAFLD (Table 1[38–44]). For example, in a study of 270 patients with MHO undergoing bariatric surgery, the presence of unsuspected NAFLD on liver histology was high, given that ∼35% of these obese patients had NAFLD, ∼10% NASH and ∼10% advanced fibrosis [38]. In a large cohort study of South Korean individuals, Sung et al. showed that the prevalence of ultrasound-defined NAFLD amongst MHO individuals was 45% [39]. More interestingly, in a cohort of 77,425 South Korean individuals who were metabolically healthy and free from NAFLD on ultrasonography at baseline, Chang et al. found that increasing BMI was independently associated with an increased incidence of NAFLD over a mean follow-up of 4.5 years, therefore suggesting that the obese phenotype, regardless of the MetS abnormalities, might increase the risk of developing NAFLD [40]. Also, in a large-scale cohort study of ∼60,000 Korean adults (22% had MHO) with imaging-defined NAFLD, Kim et al. reported that BMI was independently associated with worsening of liver fibrosis (as detected non-invasively by the NAFLD fibrosis score) in both MHO and MUO individuals over a median follow-up of 7.7 years [44]. Interestingly, in this study ∼70% of metabolically healthy individuals (irrespective of their obesity status) became metabolically unhealthy over the follow-up period. When these temporal changes in metabolic health status were considered, the increased risk of developing advanced liver fibrosis was restricted only to individuals belonging to the “higher than normal” categories of BMI and waist circumference, thereby suggesting that metabolic health status is not a static condition and that a healthy metabolic profile at baseline does not necessarily guarantee favorable long-term liver outcomes in MHO individuals [44]. In this context, some Asian studies also suggested that NAFLD is independently associated with future conversion from metabolically healthy status to metabolically abnormal phenotype [45,46], with up to 25–30% of MHO individuals who can convert to the MUO phenotype over a median follow-up of 5 years, thereby supporting a major (although poorly characterized) role of NAFLD in the transition from MHO to MUO.
Principal cross-sectional and longitudinal studies that have examined the association between metabolically healthy (MHO) or unhealthy obesity (MUO) and risk of NAFLD.
| Author, Ref. | Study characteristics | MHO definition | NAFLD diagnosis | Statistical adjustment(s) | Main findings |
|---|---|---|---|---|---|
| Haskins et al. [38] | Cross-sectional study: 270 United States MHO patients who underwent bariatric surgery with intra-operative liver biopsy from 2008 to 2015 | Not having any MetS components | Biopsy | None | A significant proportion of MHO patients had unsuspected NAFLD |
| Sung et al. [39] | Cross-sectional study: 14,384 South Korean adults from an occupational cohort | Not having any MetS components | Ultrasonography | Age, sex, smoking, alcohol intake, exercise, plasma lipids, HOMA-IR, hs-CRP, thyroid function | MHO subjects had increased risk of NAFLD, but an attenuated risk of subclinical cardiac atherosclerosis (on cardiac computed tomography). In contrast, MUO subjects and metabolically unhealthy non-obese subjects had increased risk of NAFLD and subclinical cardiac atherosclerosis |
| Chang et al. [40] | Longitudinal cohort study: 77,425 South Korean adults free of NAFLD and metabolic abnormalities at baseline. Mean follow-up: 4.5 years | Not having any MetS components and having a HOMA-IR <2.5 | Ultrasonography | Age, sex, center, year of screening exam, smoking, alcohol intake, exercise, education level | In a large cohort of strictly defined metabolically healthy men and women, BMI categories were progressively associated with increased incidence of NAFLD |
| Huh et al. [41] | Cross-sectional study: 2,198 Korean adults without chronic liver disease undergoing a medical health check-up | Not having any MetS components | Ultrasonography and transient elastography | Age, sex, smoking, ALT, GGT, LDL-cholesterol, uric acid, ferritin, hs-CRP | MHO was associated with increased prevalence of NAFLD and advanced fibrosis |
| Gutierrez-Grobe et al. [42] | Cross-sectional study: 428 adults with NAFLD | Not having any MetS components | Ultrasonography and transient elastography | None | MHO patients had a lower prevalence of advanced liver fibrosis than those with MUO |
| Ampuero et al. [43] | Cross-sectional study: 1,058 Spanish patients with biopsy-proven NAFLD | Not having any MetS components | Biopsy | Age, sex, HOMA-IR, platelet count, serum AST | Both MHO and MUO were independently associated with increased risk of NASH and significant fibrosis |
| Kim et al. [44] | Longitudinal cohort study: 59,957 South Korean adults with NAFLD. Median follow-up: 7.7 years | Not having any MetS components and having a HOMA-IR <2.5 | Ultrasonography; advanced fibrosis by NAFLD fibrosis score (NFS) | Age, sex, center, year of screening exam, smoking, alcohol intake, regular exercise, education level, history of CVD, plasma lipids, fasting glucose, HOMA-IR, hs-CRP | Excess adiposity was associated with worsening of NFS, regardless of metabolic abnormalities |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CVD, cardiovascular disease; GGT, Gamma-glutamyl transferase; HOMA-IR, homoeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C reactive protein; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Studies also reported that MHO is associated with higher proportion of advanced forms of NAFLD [38,41–43]. However, the prevalence of NASH and advanced fibrosis among MHO patients seems to be much lower than among MUO individuals [42,43]. In a study of ∼1100 Spanish patients with biopsy-confirmed NAFLD, Ampuero et al. showed that, compared to non-obese individuals, MHO patients had a ∼2-fold increased risk of having NASH, whereas those with MUO had a ∼3.5-fold increased risk of NASH after adjustment for age, sex, HOMA-IR, platelet count and serum transaminase levels [43]. Similar findings were observed for the risk of significant liver fibrosis [43].
Collectively, current evidence suggests that MHO patients have a higher risk of NAFLD and liver disease progression than NWMH individuals. However, this risk is generally lower than that observed in MUO patients. This suggests a greater adverse impact of coexisting MetS abnormalities than obesity per se on the severity of NAFLD. However, given that MHO is not a stable state and NAFLD itself may predict the transition from MHO to MUO, we believe that greater efforts should be made to identify NAFLD in all obese individuals even if they appear to be “metabolically healthy”.
Recent studies also supported a role of genetic factors in the relationship between MHO/MUO and risk of NAFLD [47,48]. Evidence suggests an interaction-effect of specific NAFLD-related genetic polymorphisms and obesity. In particular, a strong interaction-effect has been reported between the PNPLA3 rs738409 polymorphism and obesity [49–51]. In a multi-national cohort study, Stender et al. showed that, compared to those carrying the PNPLA3 C/G or C/C genotypes, the carriers of the PNPLA3 G/G genotype had a risk of developing cirrhosis, which ranged from ∼2.5-fold if they were lean to ∼6-fold if they were obese, thereby suggesting that adiposity amplifies the genetic risk of NAFLD conferred by multiple loci [49]. The authors also reported an interaction-effect between adiposity and TM6SF2 polymorphism, but this effect was much lesser than that of PNPLA3 polymorphism [49]. Interestingly, in an exome-wide association study involving almost 300,000 individuals, Liu et al. showed that polymorphisms in genes involved the hepatic production of triglyceride-rich lipoproteins (i.e. TM6SF2 and PNPLA3) were associated with higher liver fat content (LFC), higher risk of T2DM, and lower risk of coronary artery disease, whereas triglyceride-lowering alleles involved in peripheral lipolysis (i.e. lipoprotein lipase and angiopoietin-like protein 4 genes) had no effect on LFC but decreased risks for both T2DM and coronary artery disease [51].
3PathophysiologySchematically, there are two chief aspects to consider: (a) the mechanisms of liver injury in patients with obesity and (b) the mechanisms and risk factors for the development of obesity-related metabolic abnormalities (i.e. MUO) in those who have NAFLD.
3.1Mechanisms of liver injury in obesityThe widespread axiom that “fatty people have fatty livers” does not suggest what begets what. Recent studies identified key molecular mediators (as specified below), which may contribute to the development and progression of NAFLD in patients with obesity.
Glucocorticoids may facilitate all steps of NAFLD pathogenesis by stimulating adipose tissue lipolysis with free fatty acid overflow into the liver and promoting hepatic de novo lipogenesis [52]. Lutz et al. found that pharmacological blockade of 11β-hydroxysteroid-dehydrogenase 1 (11β-HSD1) (i.e. the enzyme catalyzing the conversion of cortisone to cortisol in peripheral tissues) significantly improved LFC without any changes of insulin resistance [53]. The authors also found that increased LFC more than visceral fat mass, was strongly associated with three single-nucleotide polymorphisms in the 11β-HSD1-coding gene [53]. These findings suggest that a shared genetic background may influence the risk of both visceral obesity and NAFLD.
Fibroblast growth factor-21 (FGF21) may exert beneficial effects on both lipid metabolism and the liver. In animal models, the administration of exogenous FGF21 leads to improvements in NAFLD, insulin resistance and reduction of obesity [54]. A small phase 2a randomized controlled trial found that a 16-week treatment with subcutaneously administered pegbelfermin (i.e. a PEGylated human FGF21 analog) significantly reduced LFC in overweight/obese patients with biopsy-proven NASH [55].
Neurotensin is a small peptide released by specialized jejuno-ileal entero-endocrine cells following fat ingestion that may physiologically facilitate the absorption of lipid substrates. Higher circulating levels of pro-neurotensin are found in obese than in non-obese subjects, and higher baseline levels of plasma pro-neurotensin predict a doubling of the risk of developing obesity later in life [56]. Moreover, in severely obese individuals undergoing bariatric surgery higher plasma pro-neurotensin levels were also associated with more severe histological forms of NAFLD [57].
An expanded and inflamed (dysfunctional) visceral adipose tissue is one of the major risk factors of NAFLD development and progression [3]. Experimentally, it has been demonstrated that surgical ablation of inflamed epididymal adipose tissue may attenuate NASH development in C57BL/6J mice with high-fat diet-induced obesity [58]. Du Plessis et al. have also shown that visceral and subcutaneous adipose tissues from obese patients with NAFLD or NASH have an increased expression of pro-inflammatory genes, and adipose tissue macrophages producing increased levels of proinflammatory cytokines compared to adipose tissues from obese controls without NAFLD [59].
As previously discussed, some genetic polymorphisms are likely to reduce the threshold at which obese individuals may develop NAFLD or NASH. For example, Tai et al. showed that the peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PPARGC1A) rs8192678 genotype and the PNPLA3 rs738409 genotype exerted additive negative effects on risk of NASH in severely obese patients [60]. However, the complete identity of “genetics-related NAFLD” and “MetS-related NAFLD” in terms of associated cardiovascular risk remains to be proven. For example, in a study involving individuals carrying the mutant PNPLA-3 polymorphism, MetS patients with the wildtype PNPLA-3 polymorphism and control subjects, Di Costanzo et al. found that only “metabolic NAFLD” (but not “genetic NAFLD” owing to the PNPLA-3 genotype) was associated with increased carotid intima-media thickness and increased prevalence of carotid atherosclerotic plaques [61].
These findings are in agreement with studies showing a dissociation of LFC from de novo lipogenesis (i.e. a key feature in NAFLD pathogenesis) in PNPLA3 risk allele carriers with NAFLD [62], who had normal insulin sensitivity as a result of their hepatic lipidomic profile featuring an unaltered proportion of diacylglycerol FA18:1 lipid species [63]. In line with this, another study found that carriers of the PNPLA3 polymorphism, despite having higher LFC, had better insulin sensitivity and lower expression of the proinflammatory tumor necrosis factor-alpha gene than those who did not [64]. Similarly, dissociation between LFC and insulin resistance has also been reported in patients with familial hypobetalipoproteinemia [65,66].
In the course of the natural history of NAFLD, only a minority of patients develop advanced fibrosis, cirrhosis or HCC. Recent studies have identified key molecular mediators of increased hepatic fibrogenesis and carcinogenesis specifically involved in a fatty liver “milieu”. Petta et al. by evaluating a series of patients with biopsy-proven NASH and steatosis-free controls found that the rs3480 A>G polymorphism of the fibronectin type III domain-containing protein 5 gene (encoding for the myokine irisin) exerted protective effects on advanced liver fibrosis, while the expression of irisin was associated with greater severity of NAFLD and deposition of extracellular matrix [67]. These findings suggest that genetic factors and irisin may play important roles in regulating hepatic fibrogenesis. Another study found that obese patients with cirrhosis alone or in combination with HCC had higher plasma tenascin-C levels (i.e. an extracellular matrix protein playing a role in fibrogenesis and carcinogenesis) compared to patients with less severe liver disease or control subjects [68]. Finally, in obesity-driven and NASH-driven HCC mouse models, metabolic reprogramming mediated by the down-regulation of carnitine palmitoyl-transferase-2 enabled HCC cells to escape lipotoxicity and promoted hepatocarcinogenesis via acylcarnitine accumulation [69].
3.2Mechanisms and risk factors for the development of MUO in NAFLDNAFLD is actively involved in the development of obesity-related metabolic abnormalities. In fact, NAFLD exacerbates hepatic/systemic insulin resistance, predisposes to atherogenic dyslipidemia and causes the release of several proinflammatory and vasoactive mediators that may promote the development of obesity-related cardiometabolic complications [34,70,71]. Which modifiers and mechanisms are specifically associated with MUO in those with NAFLD?
It is known that the severity of obesity strongly predicts the development of obesity-related metabolic complications (MUO). For example, the risk of developing T2DM/prediabetes in patients with NAFLD is associated with increasing BMI values. Liu et al. by retrospectively evaluating data of 18,507 Chinese men found a strong and independent association between NAFLD and risk of T2DM/prediabetes, which was closely related to temporal changes in BMI [72]. Although, these results need to be confirmed in women, it is clear that there is a close link between innate immunity and metabolism and that bariatric surgery may improve obesity-related metabolic disorders, possibly through multiple metabolic and pro-inflammatory pathways, including the type I interferon (IFN) signaling, which is critical in modulating the inflammatory response triggered by infectious stimuli [73]. Accordingly, Wieser et al. showed that improved metabolic control in obese patients after laparoscopic adjustable gastric banding was associated with increased expression of type I IFN-regulated genes in adipose tissue and liver [74].
The intake of certain nutrients may make Individuals with obesity develop dysmetabolic abnormalities. For example, dietary fructose intake increases de novo lipogenesis, promotes atherogenic dyslipidemia, worsens insulin resistance, and increases visceral adiposity in individuals with obesity [75]. In agreement with this notion, the consumption of sweetened beverage increases the risk of NAFLD in overweight/obese individuals [76].
NAFLD and cardio-respiratory fitness are mutually inter-related to each other [77]. On this background, Argo et al. showed that patients with NASH had an aerobic power and capacity, which were similar to those of sedentary control subjects. Interestingly, fitness was also similar in obese compared to overweight subjects and was not associated with visceral obesity or histological NAFLD activity score [78]. These findings suggest that poor cardio-respiratory fitness may be a factor promoting MUO in patients with NAFLD/NASH. Whether physical training programs will positively influence the conversion from MUO to MHO and improve liver histology in obese patients with NAFLD via improved aerobic capacity remains to be definitely proven.
Insulin resistance contributes to the occurrence of MUO in obese individuals. Platelet-derived growth factor-α (PDGFA) plays a key role in this occurrence. Using DNA methylome and transcriptome analyses of liver biopsies from obese individuals, Abderrahmani et al. found that hypo-methylation at a CpG site in the PDGFA and PDGFA over-expression was associated with an increased risk of insulin resistance, T2DM and NAFLD [79]. These authors also found that, once secreted, PDGFA further stimulated its own expression through protein kinase C activity and contributed to insulin resistance through decreased expression of both insulin receptor and insulin receptor substrate-1 [79]. Of note, blockade of PDGFA by using PDGF receptor inhibitors and metformin restored hepatocyte insulin sensitivity, opening new therapeutic avenues against T2DM and possibly NAFLD [79]. Collectively, data support the contention that PDGFA may link NAFLD with MUO.
Increased levels of serum uric acid (SUA) are an emerging risk factor for NAFLD [80]. In a study involving ∼10,000 Chinese adults, Zhang et al. showed a synergistic interaction between elevated SUA levels and obesity in increasing the risk of having NAFLD and hypertriglyceridemia [81]. Whether SUA-lowering treatment can decrease the risk of developing NAFLD in individuals with obesity needs to be evaluated with appropriate randomized controlled trials.
Bile acids are now considered to be important signaling molecules which control energy homeostasis and whose concentrations are altered in patients with obesity or NASH [82]. Transcriptome analyses of livers from obese individuals have shown that increased serum bile acid concentrations are associated with greater insulin resistance but not with histologic necro-inflammatory lesions of NASH [82]. Given that NASH exhibits an altered bile acid profile [83], it is conceivable that bile acids mediated the association of NAFLD with MUO.
Genetic and epigenetic factors are believed to account for the large inter-individual heterogeneity of the NAFLD phenotype [80]. In a cross-sectional study of 40 twin-pairs residing in Southern California, Zarrinpar et al. reported that the inter-twin discordance in LFC (assessed by magnetic resonance imaging) may be largely explained by inheritable miRNAs [84]. Further insight into the role of specific miRNAs has been provided by the experimental findings of the study by Hanin et al. These authors found that miR-132 was a key regulator of hepatic lipid homeostasis and that injecting diet-induced obese mice with anti-miR-132 oligonucleotides, but not suppressing its individual targets, reversed the hepatic miR-132 excess and hyperlipidemic phenotype in these mice [85]. Therefore, specific microRNA patterns may potentially account for NAFLD being associated with the progression to MUO.
4ConclusionIn the last years, the concept of MHO has gained considerable scientific interest. MHO is a complex, emerging phenotype (affecting up to ∼25–30% of obese patients) with risks intermediate between metabolically healthy individuals with normal-weight and individuals who are obese and metabolically unhealthy (as summarized in Fig. 1).
Compared to individuals who are normal weight and metabolically healthy, patients with obesity are at greater risk of all-cause mortality, cardiovascular events and NAFLD even in the absence of coexisting MetS features, thus suggesting that there is no healthy pattern of increased weight. However, compared to their counterparts with MUO, patients with MHO have lower risk of all-cause and cause-specific mortality.
Better understanding of the factors that distinguish individuals with MUO and MHO may produce new insights into mechanisms responsible for obesity-related metabolic dysfunction and disease. To date, the precise underlying mechanisms responsible for the divergent effects of obesity on metabolic health are not known [13], but experimental animal studies suggest that differences in adipose tissue biology in response to weight gain may induce or prevent systemic metabolic dysfunction. It is likely that there is an important genetic contribution to the metabolic phenotype in people with obesity. However, future studies are required to better understand the transition from MHO to MUO phenotypes and whether, and to which extent, NAFLD, sex, ethnicity, lifestyle, genetic factors and intestinal microbiota play a role in the development or reversal of such phenotypes. Additional studies are also needed to clarify how the transition from MHO to MUO phenotypes might adversely affect the presence and severity of NAFLD. Finally, a better characterization of the shared genetic basis of NAFLD and other obesity-related metabolic disorders will help in clarifying why certain individuals with obesity do not develop NAFLD and the MetS features associated with obesity.AbbreviationsBMI
body mass index
11βHSD1111β-hydroxysteroid-dehydrogenase 1
HCChepatocellular carcinoma
FGF21fibroblast growth factor 21
HOMA-IRhomeostasis model assessment-insulin resistance
LFCliver fat content
MetSmetabolic syndrome
MHOmetabolically healthy obesity
MUOmetabolically unhealthy obesity
MHNWmetabolically healthy normal weight
MUNWmetabolically unhealthy normal weight
NAFLDnonalcoholic fatty liver disease
NASHnonalcoholic steatohepatitis
PDGFAplatelet-derived growth factor α
PNPLA3patatin-like phospholipase domain-containing protein 3
SUAserum uric acid
T2DMtype 2 diabetes mellitus
TM6SF2trans-membrane 6 superfamily member 2
Conflict of interestThe authors have no conflicts of interest to declare.





![Main differences in metabolically healthy (MHO) and metabolically unhealthy obesity (MUO) [when compared with metabolically healthy normal weight individuals (MHNW)]. Main differences in metabolically healthy (MHO) and metabolically unhealthy obesity (MUO) [when compared with metabolically healthy normal weight individuals (MHNW)].](https://static.elsevier.es/multimedia/16652681/0000001900000004/v2_202007040825/S1665268120300235/v2_202007040825/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




