Infection is a common complication of liver failure. Serum inflammatory markers used to diagnose infection have sufficient diagnostic sensitivity but low specificity. This study aimed to improve the early diagnosis of infections in liver failure patients by developing a diagnostic model and evaluating its predictive ability.
Patients and MethodsA retrospective analysis of clinical data from liver failure patients. Cases were divided into infected and non-infected groups according to their clinical diagnosis. Nine infection-related predictors (age, body temperature, neutrophil ratio (NE%), procalcitonin (PCT), C-reactive protein (CRP), lactic acid (Lac), serum albumin (Alb), model of end-stage liver disease (MELD) score, and sequential organ failure assessment (SOFA) score) were included in multivariate logistic regression analysis. The diagnostic model was validated, and the receiver operating characteristic (ROC) curve was used to analyze its predictive accuracy.
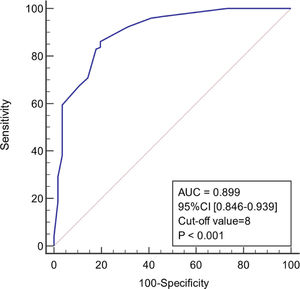
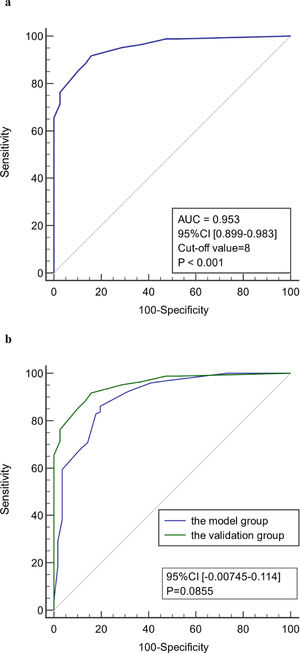
ResultsIn the model group, multivariate logistic regression analysis showed that age, body temperature, PCT, CRP, Lac, and SOFA score were independent predictors of infection associated with liver failure (P < 0.05). The area under the ROC curve (AUC) of the model was 0.899 (95% confidence interval [CI] 0.846–0.939), and the sensitivity and specificity were 86.2% and 80.4%, respectively. The AUC for the validation group was 0.953 (95% CI 0.899–0.983), and the sensitivity and specificity were 91.7% and 84.2%, respectively.
ConclusionsThis study reports a model for early diagnosis of infection in liver failure patients. The model had high overall accuracy and showed good reproducibility and reliability in patients from different centers in the same region.
Infection is a common complication of liver failure that can cause brain edema, hepatic encephalopathy, hyponatremia, hepatorenal syndrome, hemorrhage, and hepatopulmonary syndrome, leading to multiple organ dysfunction and a poor prognosis in liver failure patients. The inflammatory cytokines cascade associated with liver failure is closely related to the occurrence of infection [1]. Therefore, different methods and detection indicators are used to monitor the pathophysiological status of patients at various stages of liver failure. It is important to identify inflammatory indicators with higher sensitivity and specificity for the early stage of infection associated with liver failure. However, infection is difficult to diagnose using only a single inflammatory indicator, and a comprehensive evaluation is needed. Recent studies have combined a variety of serological indicators to improve infection diagnosis [2–3]. This study aims to improve the early diagnosis of infection in patients with liver failure by combining common clinical indicators and developing a diagnostic model.
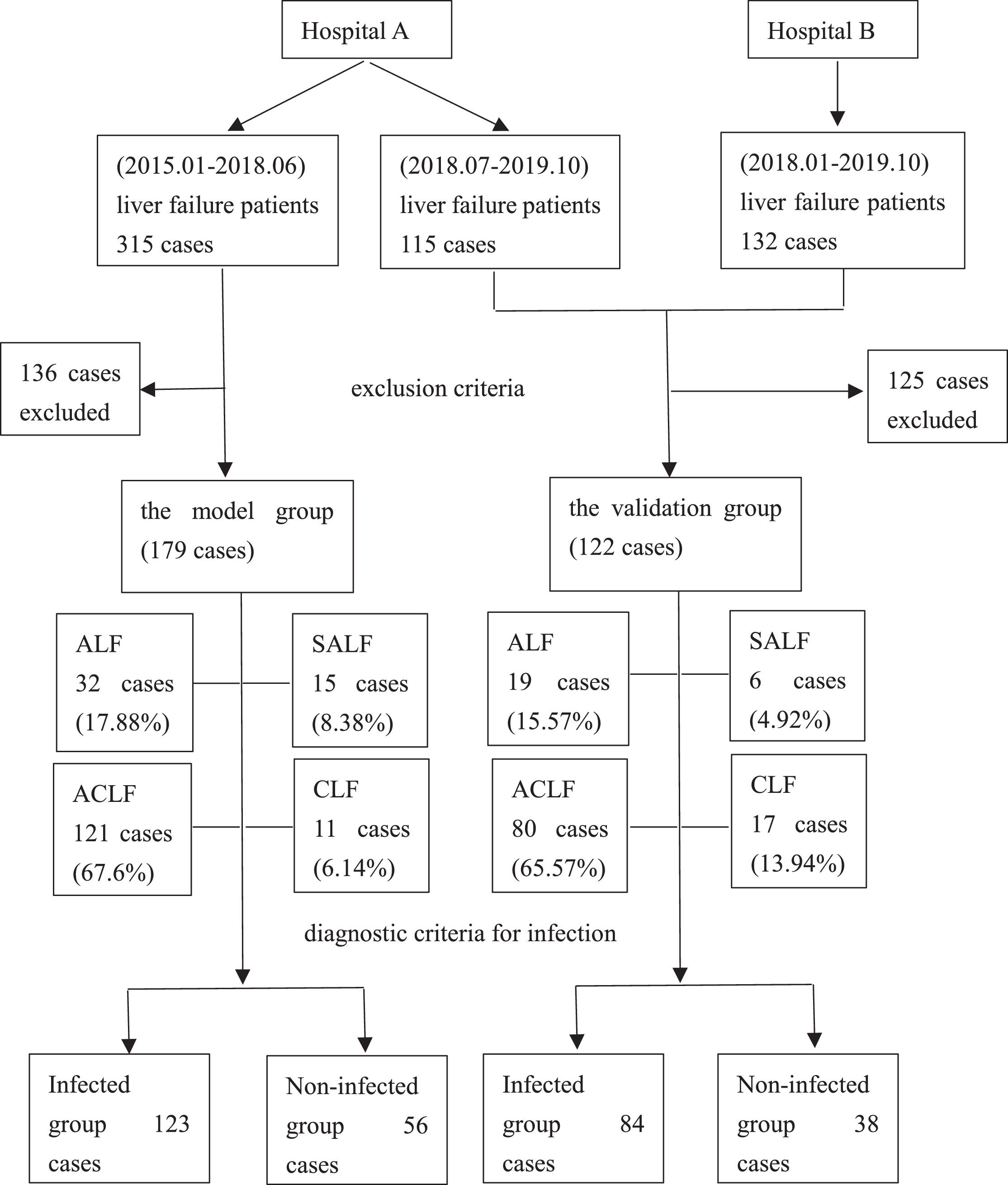
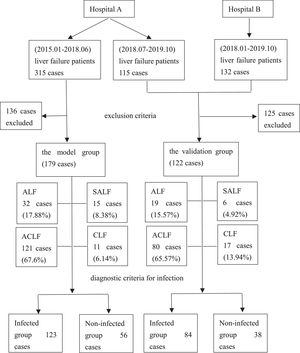
2Methods2.1Study designPatients with liver failure were enrolled. Liver failure was diagnosed according to the “Guidelines for Diagnosis and Treatment of Liver Failure” [4–6]. The exclusion criteria were as follows: patients with liver failure who were repeatedly admitted to the hospital for a short period, age <18 years, incomplete clinical data, hospitalization time <48 h, combined immunodeficiency disease, pregnancy and lactation. Finally, 179 cases were used for developing the model, and 122 cases were utilized for validating the diagnostic model from two centers. The model group and the validation group were divided into infected and non-infected groups according to the diagnostic criteria for infection, respectively. The patient enrollment process is shown in Figure 1.
2.2Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of the First Affiliated Hospital of Soochow University (No.:360) and Taicang First People's Hospital (No.:KY-2021-270).
2.3Diagnostic criteria for infectionThe diagnostic criteria for infection were based on past medical history, clinical symptoms and signs, laboratory tests (blood analysis, urinalysis, urine culture, stool culture, throat swab/sputum smear culture, ascites biochemistry and culture), and imaging examination. The common national clinical standards have been reported previously [7–11]. Spontaneous bacterial peritonitis (SBP): peritonitis clinical symptoms, polymorph nuclear leukocyte (PMN) count in ascites ≥250/mm3 (0.25 × 109/L); secondary surgical peritonitis was excluded; ascites culture was positive or negative. Lung infection: clinical symptoms associated with pneumonia (cough, sputum expectoration or exacerbation of symptoms with purulent sputum, with or without chest pain/dyspnea/hemoptysis, fever, and signs of lung consolidation), white blood cell (WBC) count > 10 × 109/L or < 4 × 109/L, with or without a neutrophil shift to the left, and chest imaging with exudation or consolidation, with or without pleural effusion. Urinary tract infection: abnormal urine sediment (>10 leukocytes/visual field), positive urine culture, or if the culture is negative, the leukocyte count/visual field is uncountable. Intestinal infection: diarrhea with WBC-positive stool or stool culture to identify pathogens. Biliary tract infection: cholestasis, right upper abdominal pain and/or jaundice, and imaging examination showing biliary obstruction. Spontaneous bacteremia: positive blood culture, septicemia. Secondary bacteremia: catheter-associated infection (positive blood and catheter culture), bacteremia within 24 hours after invasive surgery, severe infection in other organs. Skin and soft tissue infections: clinical signs of infections associated with skin swelling, erythema, heat, and tenderness.
2.4Research methodsPatient information was collected at the time of admission, including gender, age, comorbid conditions, main diagnosis, infection site, vital signs, state of consciousness, clinical treatment, and outcome. Laboratory tests included blood analysis, coagulation function, liver function, kidney function, lactic acid (Lac), C-reactive protein (CRP), and procalcitonin (PCT). For patients in the infection group, vital signs, consciousness, blood analysis, coagulation function, liver and kidney function, Lac, CRP, and PCT within 24 hours of empirically identified infection data were collected, and the model of end-stage liver disease (MELD) and sequential organ failure assessment (SOFA) scores were calculated. For patients in the non-infected group, MELD and SOFA scores within 24 hours of admission were determined according to relevant examination and scoring standards. Finally, nine variables associated with infection were selected for analysis and modeling, including age, body temperature, neutrophil ratio (NE%), PCT, CRP, Lac, serum albumin (Alb), and MELD and SOFA scores.
The MELD scoring formula was as follows: 3.8 × ln[total bilirubin (mg/dl)]+11.2 × ln(INR)+9.6 × ln[serum creatinine (mg/dl)]+6.4 × cause (bile or alcoholic = 0, Others = 1).
2.5Statistical analysisSPSS 20.0 and MedCalc 15.1 statistical software were used for statistical analysis [12],[13]. Continuous variables were tested for normality (Kolmogorov-Smirnov), and data with a normal distribution were expressed as means ± standard deviation (x ± s), and t-tests were used for between-group comparisons. Non-normally distributed data were presented as medians and interquartile ranges [M (P25, P75)], and the Wilcoxon signed-rank test was used for between-group comparisons. The χ2 test was used to compare between-group enumeration data. The receiver operating characteristic (ROC) curve was graphed using MedCalc15.1 statistical software with the value corresponding to the maximum Youden index as the critical value (cut-off value). For the predictive variables, the cut-off value or clinical reference boundary value was used as the dividing point to convert continuous variables into binary variables, represented as equal weight virtual variables and coded as 0 or 1. The reference category corresponding to the normal range was coded as 0. Collinearity analysis in multiple linear regression was used to determine collinearity between the predictors. Tolerance <0.1 and a variance inflation factor (VIF) >5 were indicators of collinearity. In univariate analysis, variables with P < 0.05 were included in the multivariate logistic regression analysis to identify independent predictors of infection in patients with liver failure. The selected independent predictors were included in the multivariate logistic regression to develop a diagnostic model for infection. The ORx value of any predictor variable in the model (rounded up) was defined as the fixed score (B) in the scoring system, with an independent variable coded value of 1 reflecting an increase in the risk of infection. The score of the remaining predictive variables was ORi/ORx × B when taking the value coded as 1 [14]. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate the model, and P > 0.05 indicated a good fit. Subsequently, the model was validated in an independent set of cases. The ROC curve, area under the ROC curve (AUC), Jordan index, sensitivity, specificity, likelihood ratio, and cut-off value were used to analyze the ability of the model to predict infection associated with liver failure. P < 0.05 was considered statistically significant.
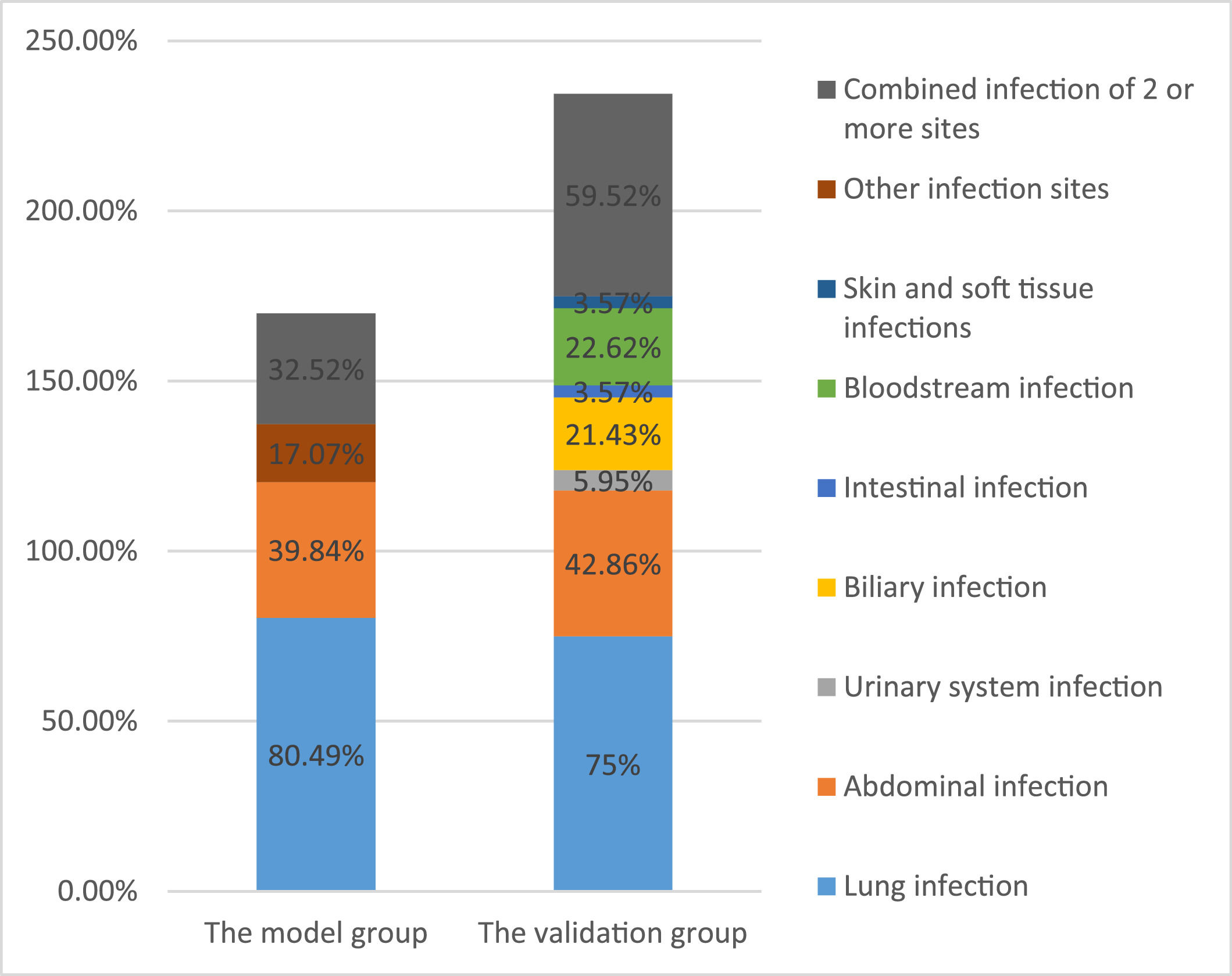
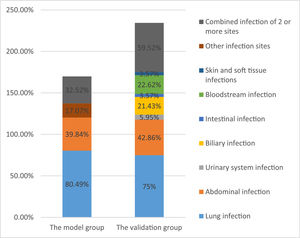
3Results3.1Patient characteristics in the model groupThe average age of 179 patients with liver failure was 53 ± 15 years. Among the patients with liver failure, acute-on-chronic liver failure was most common, accounting for 121 cases (67.60%), 41 (22.91%) were associated with malignant tumors, and 29 (16.20%) were associated with diabetes. Among the patients, 113 (63.13%) had ascites, 80 (44.69%) had hepatic encephalopathy, 28 (15.64%) had gastrointestinal bleeding, and 38 (21.23%) had hepatorenal syndrome. Bioartificial liver support was performed in 75 patients (41.90%) during clinical treatment, and hormonal treatment was used in 78 patients (43.58%). A comprehensive evaluation identified 123 cases of infection associated with liver failure, with an infection rate of 68.72%. The main infection sites were the lungs (n = 99, 80.49%) and the abdominal cavity (n = 49, 39.84%). Other infection sites included the urinary system, biliary tract, and intestinal tract (n = 21,17.07%). Forty patients (32.52%) had infections at two or more sites (Figure 2). Of the 179 patients with liver failure, 122 died, with an overall hospital mortality rate of 68.16%. Patient mortality in the infected group was significantly higher than that in the non-infected group (Table 1).
Patient characteristics of the model group
| Item | Infected group (n = 123) | Non-infected group (n = 56) | Statistics | P-value |
|---|---|---|---|---|
| Age (year) | 55 ± 15 | 49 ± 16 | -2.205 | 0.029 |
| Classification of liver failure [n (%)] | ||||
| ALF | 21 (17.07) | 11 (19.64) | 0.173 | 0.677 |
| SALF | 12 (9.76) | 3 (5.36) | 0.482 | 0.488 |
| ACLF | 83 (67.48) | 38 (67.86) | 0.003 | 0.960 |
| CLF | 7 (5.69) | 4 (7.14) | 0.002 | 0.969 |
| Comorbidity [n (%)] | ||||
| Malignant tumor | 30 (24.39) | 11 (19.64) | 0.491 | 0.483 |
| Diabetes | 24 (19.51) | 5 (8.93) | 3.175 | 0.075 |
| Major complications [n (%)] | ||||
| Ascites | 92 (74.80) | 21 (37.50) | 22.997 | <0.001 |
| Hepatic encephalopathy | 55 (44.72) | 25 (44.64) | 0.000 | 0.993 |
| Gastrointestinal bleeding | 21 (17.07) | 7 (12.50) | 0.610 | 0.435 |
| Hepatorenal syndrome | 32 (26.02) | 6 (10.71) | 5.388 | 0.020 |
| Clinical treatment [case (%)] | ||||
| Artificial liver support | 56 (45.53) | 19 (33.93) | 2.127 | 0.145 |
| Hormone use | 52 (42.28) | 26 (46.43) | 0.270 | 0.603 |
| Outcome [n (%)] | ||||
| Improved | 30 (24.39) | 27 (48.21) | 10.063 | 0.002 |
| Death | 93 (75.61) | 29 (51.79) | ||
| Fever [n (%)] | 56 (45.53) | 8 (14.29) | 16.352 | <0.001 |
| PCT (ng/mL) | 1.27 (0.72–3.10) | 0.55 (0.32–0.76) | -6.481 | <0.001 |
| CRP (mg/L) | 13.92 (11.08–14.75) | 10.51 (5.65–13.70) | -4.858 | <0.001 |
| NE% | 0.83 (0.75–0.89) | 0.80 (0.65–0.85) | -3.533 | <0.001 |
| Alb (g/L) | 31.26 ± 5.30 | 32.60 ± 5.03 | 1.547 | 0.124 |
| Lac (mmol/L) | 1.70 (1.40–2.10) | 0.90 (0.80–1.40) | -6.473 | <0.001 |
| SOFA (points) | 10 (8–12) | 7 (5–9) | -5.204 | <0.001 |
| MELD (points) | 22.91 (19.68–29.64) | 20.56 (17.58–27.24) | -2.131 | 0.033 |
ALF, acute liver failure; SALF, subacute liver failure; ACLF, acute-on-chronic liver failure; CLF, chronic liver failure; PCT, procalcitonin; CRP, C-reactive protein; NE%, neutrophil ratio; Alb, serum albumin; Lac, lactic acid; SOFA, sequential organ failure assessment; MELD, model of end-stage liver disease.
The results are presented as: mean ± standard deviation, median and interquartile ranges [M (P25, P75)], number, percent, t-statistics, chi-squared and Z-statistics.
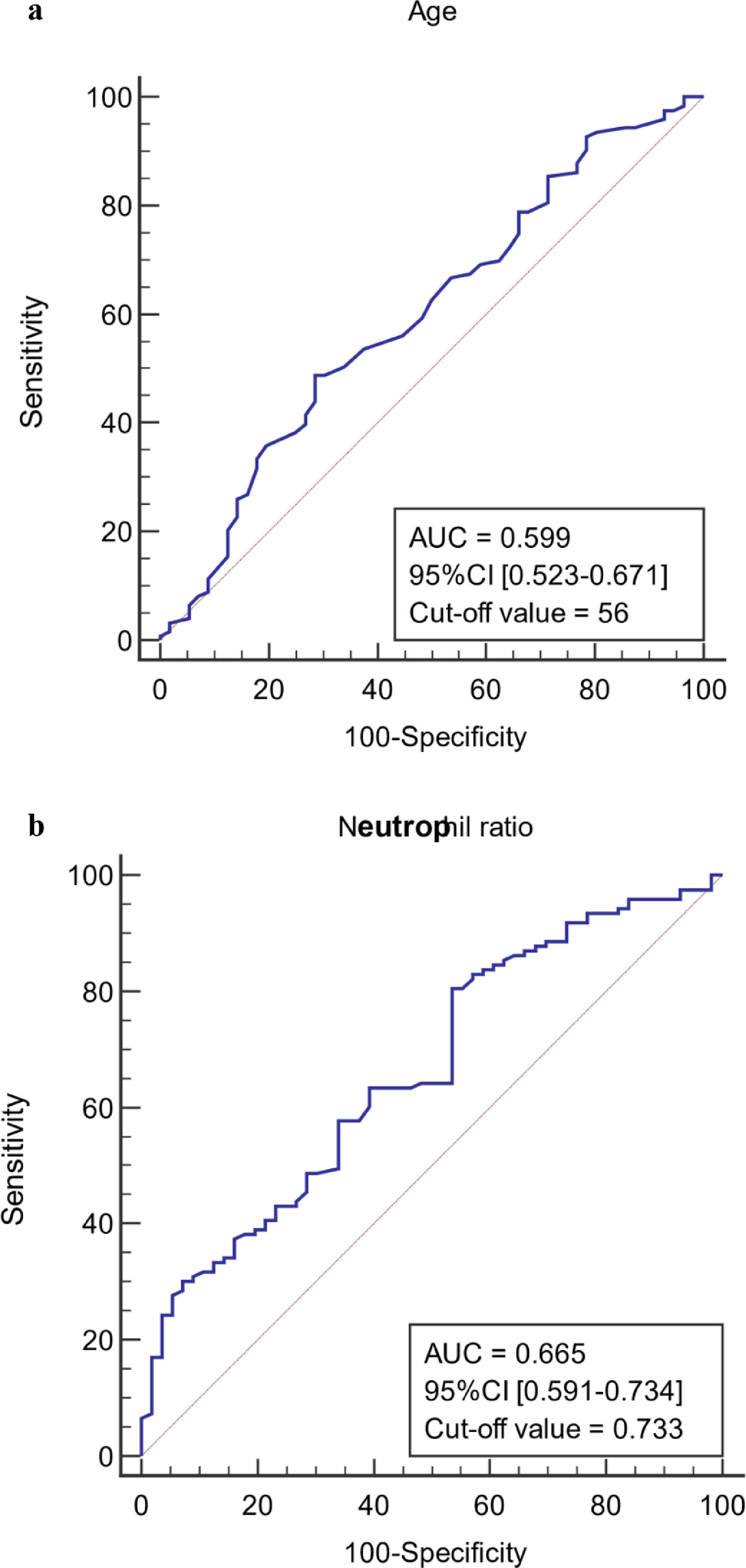
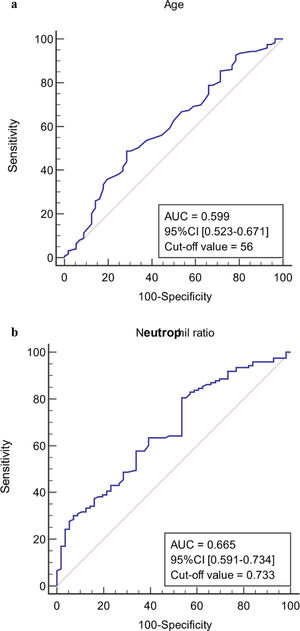
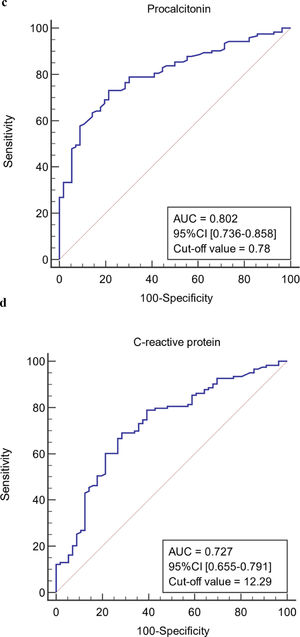
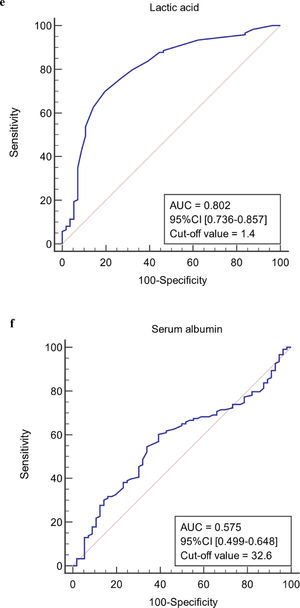
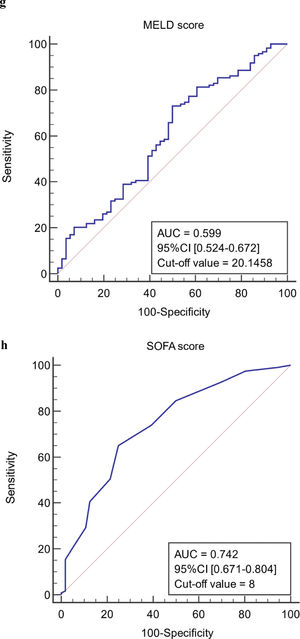
The ROC curves of continuous independent variables in the model group were generated with medcalc15.1 statistical software. The cut-off values corresponding to age, NE%, PCT, CRP, Lac, ALB, MELD, and SOFA scores were 56 years, 0.733, 0.78 ng/mL, 12.29 mg/L, 1.4 mmol/L, 32.6 g/L, 20.1458, and 8, respectively (Figure 3). Because the cut-off values for NE% and Lac (0.733 and 1.4 mmol/L) were close to the clinical reference limit (0.75 and 1.6 mmol/L), the clinical reference limit was selected as the dividing point. Body temperature was defined as an axillary temperature of 37.3 °C.
The AUC and cut-off values of nine variables associated with infection. (a-h) ROC analysis of age, neutrophil ratio, procalcitonin, C-reactive protein, lactic acid serum albumin, SOFA and MELD scores.
AUC, area under the curve; ROC, receiver operating characteristic; SOFA, sequential organ failure assessment; MELD, model of end-stage liver disease.
The collinearity statistics showed that the Tolerance of each model predictor variable is >0.1 and that the VIF is >1 and <5, indicating that there is a weak correlation between the predictive variables and that there is no obvious multicollinearity of the model. Therefore, these nine variables could be used for logistic regression.
3.4Single-factor and multi-factor analysis and development of a diagnostic model in patients with infection associated with liver failureUnivariate analysis showed that the nine indexes were closely related to infection associated with liver failure (P < 0.05). The nine variables were included in multivariate logistic regression analysis. The results showed that age, body temperature, PCT, CRP, Lac, and SOFA score were independent predictors of infection in patients with liver failure (P < 0.05). The scoring model for infection diagnosis was developed using independent predictive variables (Table 2, Table 3, and Table 4). The Hosmer-Lemeshow test showed a good fit (P = 0.347 > 0.05) and a prediction accuracy of 84.9% for the model.
Univariate and multivariate analysis of infection prediction in patients with liver failure in the model group
| Predictor variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR value (95% CI) | P-value | OR value (95% CI) | P-value | |
| Age | 2.381 (1.207–4.695) | 0.012 | 2.712 (1.040–7.075) | 0.041 |
| Body temperature | 5.015 (2.190–11.482) | <0.001 | 4.258 (1.370–13.234) | 0.012 |
| NE% | 2.465 (1.271–4.777) | 0.008 | 2.184 (0.796–5.989) | 0.129 |
| PCT | 10.00 (4.711–21.227) | <0.001 | 8.676 (3.223–23.360) | <0.001 |
| CRP | 5.592 (2.792–11.200) | <0.001 | 3.518 (1.341–9.228) | 0.011 |
| Lac | 9.649 (3.853–24.163) | <0.001 | 3.938 (1.224–12.674) | 0.022 |
| Alb | 2.334 (1.223–4.455) | 0.010 | 2.661 (0.984–7.192) | 0.054 |
| MELD | 2.727 (1.412–5.268) | 0.003 | 0.529 (0.183–1.532) | 0.241 |
| SOFA | 5.581 (2.746–11.345) | <0.001 | 3.151 (1.121–8.858) | 0.029 |
OR, odds ratio.
Development of a diagnostic model for infection associated with liver failure
| Predictor variable | Coefficient | OR value | 95% CI | P-value | Fixed score (B) | ORi /ORx × B (points) |
|---|---|---|---|---|---|---|
| Age | 1.036 | 2.817 | 1.122–7.071 | 0.027 | 3 | 3 |
| Body temperature | 1.201 | 3.324 | 1.122–9.852 | 0.030 | 4 | |
| PCT | 2.055 | 7.804 | 3.070–19.836 | <0.001 | 8 | |
| CRP | 1.188 | 3.281 | 1.337–8.050 | 0.009 | 3 | |
| Lac | 1.620 | 5.055 | 1.639–15.593 | 0.005 | 5 | |
| SOFA | 1.014 | 2.757 | 1.089–6.979 | 0.032 | 3 | |
| Constant | -2.371 | 0.093 | <0.001 | 0 |
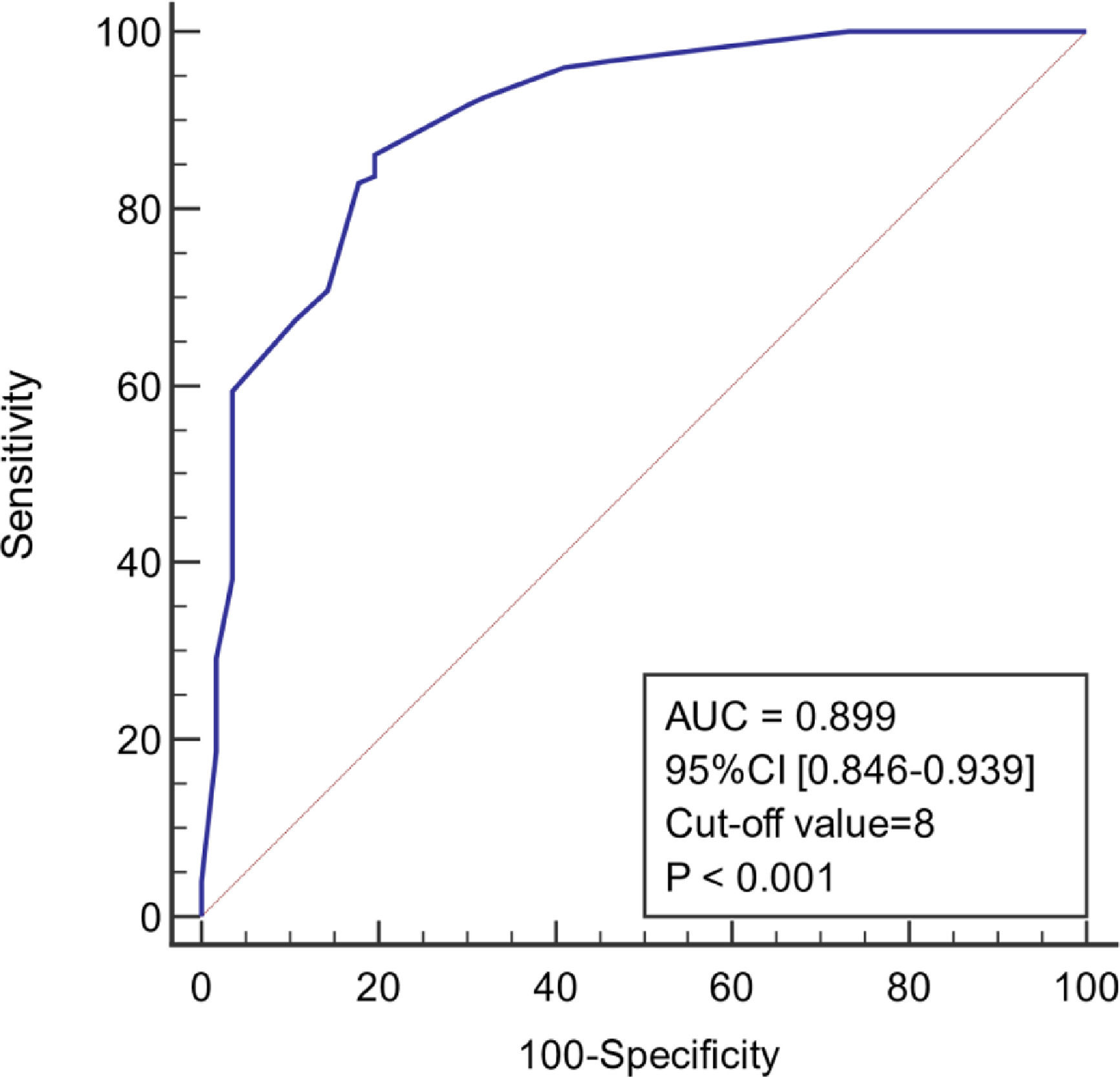
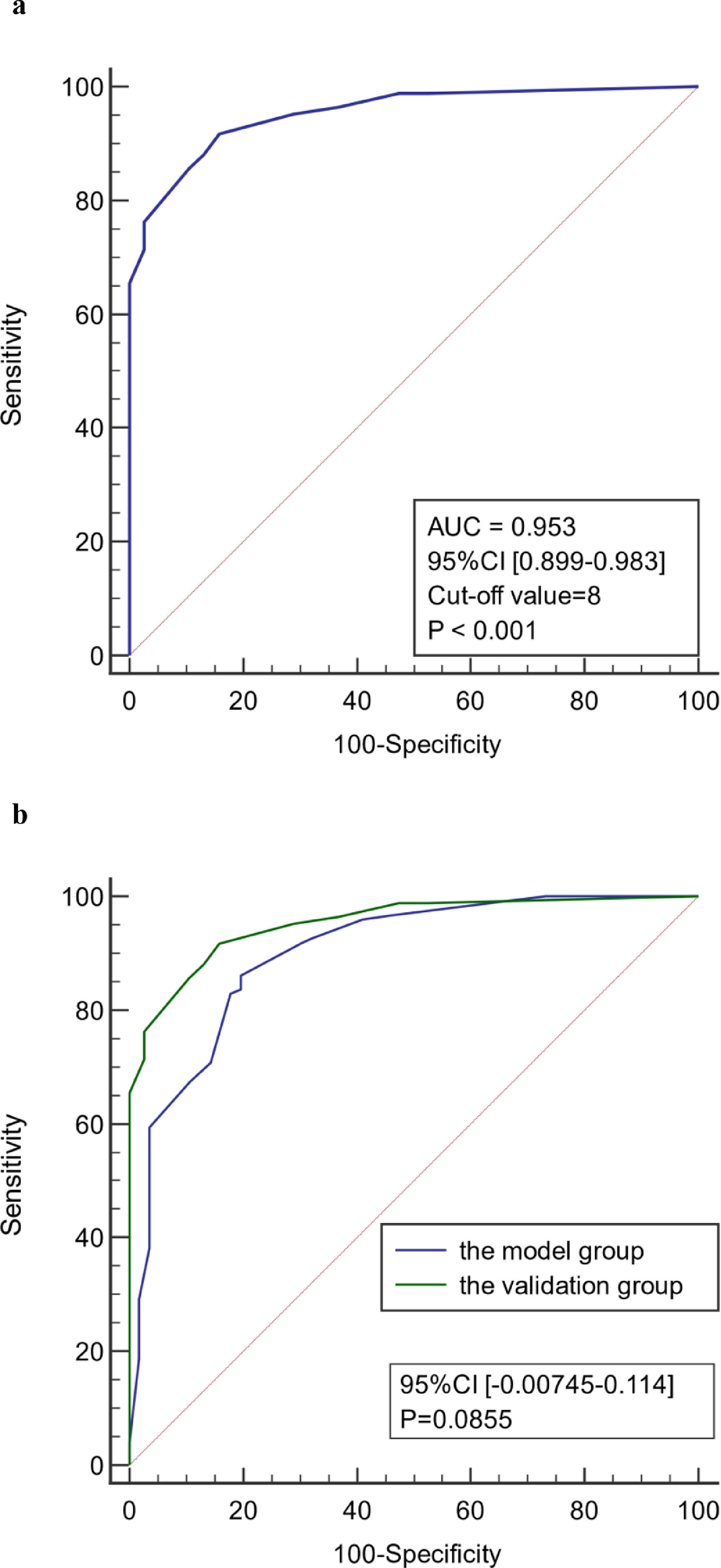
As shown in Figure 4, the diagnostic model had good predictive accuracy for the early stages of infection associated with liver failure (AUC = 0.899, 95% confidence interval [CI] 0.846–0.939), cut-off value = 8, and the sensitivity and specificity were 86.2% and 80.4%, respectively. The positive likelihood is high (4.39), and the negative likelihood is low (0.17) for overall high accuracy.
3.6Validation of the reliability of the model for early diagnosis of infection associated with liver failureCharacteristics of liver failure patients in the validation groupThe average age of 122 patients with liver failure in the validation group was 52 ± 14 years. Liver failure was classified as acute-on-chronic liver failure in 80 cases (65.57%). After a comprehensive analysis, 84 cases were shown to be associated with infection with an incidence of 68.85%, including 63 cases of pulmonary infection (75%) and 36 cases of abdominal infection (42.86%). Other infection sites included biliary (n = 18, 21.43%), urinary tract (n = 5, 5.95%), intestinal (n = 3, 3.57%), blood (n = 19, 22.62%) and skin and soft tissue (n = 3, 3.57%) infections. Fifty patients (59.52%) had infections at ≥2 sites. The overall in-hospital mortality was 55.74%, and the mortality in the infected group was significantly higher than that in the non-infected group (P < 0.05) (Table 5 and Figure 2).
Patient characteristics in the validation group
| Item | Infected group (n = 84) | Non-infected group (n = 38) | P-value |
|---|---|---|---|
| Age (years) | 56 ± 13 | 45 ± 13 | <0.001 |
| Classification of liver failure [n (%)] | |||
| ALF | 13 (15.48) | 6 (15.79) | 0.965 |
| SALF | 3 (3.57) | 3 (7.89) | 0.568 |
| ACLF | 52 (61.90) | 28 (73.68) | 0.205 |
| CLF | 16 (19.05) | 1 (2.63) | 0.032 |
| Comorbidity [n (%)] | |||
| Malignant tumor | 8 (9.52) | 2 (5.26) | 0.661 |
| Diabetes | 12 (14.29) | 5 (13.16) | 0.868 |
| Major complications [n (%)] | |||
| Ascites | 60 (71.43) | 15 (39.47) | 0.001 |
| Hepatic encephalopathy | 56 (66.67) | 16 (42.11) | 0.011 |
| Gastrointestinal bleeding | 15 (17.86) | 2 (5.26) | 0.115 |
| Hepatorenal syndrome | 26 (30.95) | 2 (5.26) | 0.004 |
| Clinical treatment [n (%)] | |||
| Artificial liver support | 59 (70.24) | 21 (55.26) | 0.107 |
| Hormone use | 40 (47.62) | 19 (50.00) | 0.807 |
| Outcome [n (%)] | |||
| Improved | 27 (32.14) | 27 (71.05) | <0.001 |
| Death | 57 (67.86) | 11 (28.95) | |
| Fever [n (%)] | 56 (66.67) | 6 (15.79) | <0.001 |
| PCT (ng/mL) | 1.36 (0.96–2.24) | 0.42 (0.30–0.68) | <0.001 |
| CRP (mg/L) | 15.36 (9.43–30.33) | 5.7 5(3.97–12.01) | <0.001 |
| Lac (mmol/L) | 1.20 (0.90–1.50) | 1.25 (0.80–1.50) | 0.846 |
| SOFA (points) | 9.00 (7.00–13.00) | 6.00 (4.00–8.00) | <0.001 |
ALF, acute liver failure; SALF, subacute liver failure; ACLF, acute-on-chronic liver failure; CLF, chronic liver failure; PCT, procalcitonin; CRP, C-reactive protein; Lac, lactic acid; SOFA, sequential organ failure assessment.
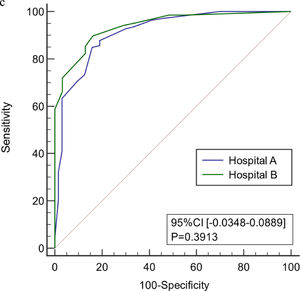
The age, body temperature, PCT, CRP, Lac, and SOFA score values of patients in the validation group were converted into binary variables and the model was used to calculate the total score for each patient. ROC curve analysis showed that the model had good predictive performance for early diagnosis of infection associated with liver failure with a AUC of 0.953 (95% CI 0.899–0.983). When the value corresponding to the maximum Youden index (0.759) was used as the critical value (8 points), the sensitivity for early diagnosis of liver failure associated with infection was 91.7%, the specificity was 84.2%, the positive likelihood ratio was 5.81, and the negative likelihood ratio was 0.099. There was no significant difference in the ROC curve between model group and validation group (P < 0.05). There was also no significant difference in the ROC curve between two centers (P < 0.05). Therefore, the model has good stability and reliability. (Figure 5)
ROC curve of the model for early diagnosis of infection. (a) The prediction of concurrent infections in the validation group. (b) Comparison of the ROC curve of model for early diagnosis of infection between model group and validation group. (c) Comparison of the ROC curve of model for early diagnosis of infection in two centers.
Microbial infection is an important precipitating factor of liver failure, and it is also a common complication of liver failure. Data from CANONIC in Europe and an Asian study show that the incidence of bacterial infections in patients with liver failure is 33% when they are admitted to the hospital and 57% during hospitalization [15–16]. The data for the model group in this study came from a single-center database, and the validation group data were from 2 centers in the same region. The infection rate and main infection sites of liver failure patients in the model group and the validation group were similar. There were minor differences from data reported previously, which may be related to data collection during hospitalization of liver failure patients, including data collected at the time of admission, as well as local differences in infection epidemiology. Studies have shown that patients with chronic liver failure associated with diabetes, upper gastrointestinal bleeding, clinical artificial liver support, and hormone treatment have a higher chance of infection [17–18]. In this study, infections were not associated with these factors, which may be related to the small number of gastrointestinal bleeding cases and the timely control of blood sugar levels during clinical diagnosis and treatment. Liver failure combined with hepatorenal syndrome was significantly associated with infection (P < 0.05).
Infection-associated predictors based on the OR 95% CI were selected for single-factor analysis of multiple related indicators to improve the reliability of the regression results and to reduce bias. These included age, body temperature, NE%, PCT, CRP, Lac, Alb, MELD, and SOFA score for multivariate logistic regression analysis modeling. Eight indicators (age, body temperature, NE%, PCT, CRP, Lac, MELD, and SOFA score) were significantly different between the infected group and the non-infected group in the univariate analysis (P < 0.05). There was no significant statistical difference in Alb between the two groups (P = 0.124). However, several other studies have shown that serum albumin is indicative of the liver's reserve and defense capabilities [19–20]. It is also closely associated with concurrent infection and prognosis of patients with end-stage liver disease. Therefore, Alb was included in this study.
After multivariate logistic regression analysis, age, body temperature, PCT, CRP, Lac, and SOFA score were selected as independent predictors of infection in liver failure patients (P < 0.05). Elderly patients are susceptible to multiple diseases. Therefore, the older the patients with liver failure, the higher their risk of infection. Studies have shown that the incidence of infection in patients with decompensated liver cirrhosis is significantly higher than that in patients aged ≥60 years [21–22]. In this study, the critical age value determined by the model was 56 years, and there was a significant difference between the infected and non-infected groups (P < 0.05). The risk of infection in patients with liver failure who were older than 56 years was 2.817 times that of those younger than 56 years.
Fever has always been regarded as a sign of infection; however, infection in elderly and frail patients can be present without fever [23]. A normal or low body temperature sometimes reflects a failure of the host's defense against infection [24]. Therefore, it is important to distinguish between infectious fever and non-infectious fever. Bossink et al. found that only peak body temperature and WBC peak can predict microbial infection in a study with 300 patients [25]. Circiumaru et al. found that fever (defined as a body temperature of 38.4°C) was associated with infection in 53% of patients [26]. Due to immune system disorders in patients with liver failure, the stages of infection and inflammation are difficult to distinguish, and body temperature cannot be used as the only indicator of infection. In addition, clinical interventions are usually performed before the body temperature reaches its peak. Therefore, we used the standard axillary temperature of 37.3 °C as the cut-off value in the model, which is a fairly low cut-off value. However, in the model group, the univariate analysis of body temperature showed a significant difference between the infected and non-infected groups (P < 0.001), and the estimated body temperature score was up to 4 points.
Both PCT and CRP are serum acute-phase response proteins, which are commonly used clinical indicators of inflammation in response to infection. However, due to the complex relationship between PCT and CRP and the liver, the clinical value of PCT and CRP in the diagnosis of liver disease associated with infection has not been established. The liver is one of the tissues that produce PCT in response to bacterial infection, leading to speculation that PCT secretion in patients with impaired liver function is decreased or not increased [27]. However, most reports indicate that in patients with advanced liver disease, baseline PCT levels can be elevated even in the absence of bacterial infection, and endotoxemia and damage-associated molecular patterns may be potential factors correlated with total bilirubin level [28]. CRP serum levels in patients with liver failure are also variable. Studies have shown that CRP levels in patients with advanced liver cirrhosis without infection and after infection control can stay elevated for long periods [29]. There are also many studies showing that CRP levels are negatively correlated with the degree of liver failure[30–31]. Even if PCT and CRP levels are affected by liver function, their early diagnostic value for infection associated with advanced liver disease has been well studied, and there are no significant differences from patients without liver failure. However, thresholds for PCT and CRP levels for the diagnosis of infection have yet to be determined [27],[29],[32]. The cut-off value for PCT in the model group in this study was 0.78 ng/mL, and the cut-off value for CRP was 12.29 mg/L. Therefore, PCT >0.78 ng/mL or CRP >12.29 mg/L were indicative of a possible infection.
Lac is an important indicator, which reflects tissue perfusion and cellular hypoxia. Recent studies have used it to assess the prognosis of sepsis, severe pneumonia, and liver failure patients [33–34]. In patients with liver failure, liver function deteriorates rapidly, liver microcirculation is impaired, and there is local and systemic inflammation of the liver. Since the liver is the main organ for removing Lac, liver failure patients have higher Lac levels than the normal threshold. When liver failure is associated with infection, especially with a lung infection, Lac level is significantly increased.
The SOFA score involves the liver, kidneys, blood coagulation, breathing, circulation, and nervous system. It can comprehensively and effectively assess the severity of organ failure in patients. It is also a recognized reliable indicator for the diagnosis and prognosis of patients with acute and severe infections. It has been widely used in clinical practice [35–36]. The critical SOFA score value in the model group in this study was 8 points.
Although the indicators discussed above are helpful for the diagnosis of infection and have been widely used in clinical practice, it is difficult to diagnose or rule out the occurrence of infection with a single indicator, and comprehensive evaluation is required. In this study, the six independent predictors of infections in liver failure patients identified in the model group were used multivariate logistic regression, and a scoring model for early diagnosis of infection was successfully developed. The scoring model included the six indicators of age, body temperature, PCT, CRP, Lac, and SOFA score with corresponding scores of 3, 4, 8, 3, 5, and 3 points. Scores less than the threshold are all 0 points. The sum of the scores was the total score (0–26 points).Data collected from two centers in the same region was used as the validation group. The model was validated with the sample size of the model group at a ratio of 68%. The results showed that the scoring model had a better predictive performance for the early stage of infection associated with liver failure. The cut-off value of the ROC curve in the validation group was the same as that in the model group. Therefore, the scores >8 was indicative of a possible infection, and the sensitivity and specificity were 91.7% and 84.2%, respectively. There was no significant difference in the ROC curve between two groups and between two centers (P < 0.05). The overall accuracy was high. It showed that the scoring model had good reproducibility and high reliability.
5ConclusionsIn summary, this study in patients with liver failure used common clinical indicators to develop a simple model for early diagnosis of infection. The diagnostic model predicted the probability of infection using a score and an integral for each indicator. The model is simple and intuitive, thereby facilitating clinical application. However, in the clinical setting, an infection diagnosis should be comprehensively evaluated in combination with the patient's medical history, clinical manifestations, imaging, and pathogen testing. Due to its special pathophysiological characteristics, we excluded patients: age <18 years, combined immunodeficiency disease, pregnancy and lactation. For patients with liver failure who were repeatedly admitted to the hospital for a short period of time, hospitalization time <48 h, and incomplete clinical data, it will bring some bias to the study results. However, whatever the clinical severity of patients, improving relevant examinations can help us diagnose in time. The diagnostic model developed in this study can be regarded as a supplementary tool to be used as a reference. In addition, this study is a retrospective analysis, and data were only collected when patients are admitted to the hospital and when signs of infection were empirically identified, while dynamic changes were not continuously detected. Moreover, the model data is from a single center. Although the validation group added data from another center in the same region, the sample size is small. Therefore, a more extensive multi-center prospective study is needed to validate the results of this study. Further, with the emergence of more effective indicators for infection diagnosis, the predictor variables in this model may be replaced, and the model will have to be continuously improved.
Data availability statementThe datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributionsConception and design: Wenhui Liu, Yonglan Pu, Chuanwu Zhu, Ailan Qin.; Literature research and data acquisition: Wenhui Liu, Chuanwu Zhu, Ailan Qin.; Data analysis/interpretation: Wenhui Liu, Ailan Qin.; Manuscript editing: Wenhui Liu, Chuanwu Zhu, Ailan Qin.; Manuscript revision/review: Wenhui Liu, Yonglan Pu, Chuanwu Zhu, Ailan Qin.; Supervision: Ailan Qin and Yonglan Pu. All authors significantly contributed to the intellectual contents of this manuscript and approved its submission.
Declaration of interestNone.
This study was supported by the Key Scientific Program of the Department of Science and Technology during the 13th “Five-Year Plan” Period of China, No.2017ZX10203201002-002.