Editado por: Marco Arrese - Pontifical Catholic University of Chile, Santiago, Chile
Más datosThe Baveno VII consensus workshop has provided several novel recommendations regarding the management of patients with clinically significant portal hypertension (CSPH). The expert panel summarized the existing data into simple clinical rules to aid clinicians in their clinical practice. The use of non-invasive tests (NITs), especially liver stiffness measurement (LSM), have gain an important role in daily practice. The use of LSM alone or in combination with platelet count can be used to rule-in and rule-out compensated advanced chronic liver disease (cACLD) and CSPH. Further decompensation events were defined as a prognostic stage associated with an even higher mortality than that associated with first decompensation. Moreover, the term hepatic recompensation was introduced in Baveno VII consensus implying a partial or complete regression of the functional and structural changes of cirrhosis after the removal of the underlying etiology. This review will summarize the reader main aspects of Baveno VII consensus regarding the use of NITs in cACLD, analyze further decompensation events, and evaluate recent recommendations for prophylaxis and management of liver decompensation events.
The development of portal hypertension is the most critical milestone in the natural history of advanced chronic liver disease. Portal hypertension is defined as an increase in portal pressure gradient between the portal vein and the inferior vena cava above 5 mmHg. Cirrhosis is by far the most common cause of portal hypertension. The appearance of variceal bleeding, ascites, hepatic encephalopathy or non-obstructive jaundice, herald the onset of decompensated cirrhosis. Patients who have not yet developed these complications have compensated cirrhosis. In cirrhosis, portal hypertension results from an increase in intrahepatic vascular resistance; being the consequence of structural (due to the presence of fibrous tissue in the sinusoids and the formation of regenerative nodules), and functional (due to sinusoidal endothelial cell dysfunction leading to vasoconstriction) abnormalities. Portal flow increased occurs secondarily maintaining and aggravating portal hypertension, despite the formation of collaterals veins. Clinically significant portal hypertension (CSPH) becomes evident when the hepatic venous pressure gradient (HVPG) increases to 10 mmHg or greater, promoting the development of gastroesophageal varices [1]. The risk of variceal hemorrhage increases with the increase in HPVG. The increase in HVPG beyond 10 mmHg leads to circulatory abnormalities, i.e. the development of splanchnic arteriolar vasodilation [2]. Ongoing splanchnic vasodilatation results in a decrease in effective arterial blood volume causing systemic hypotension and activation of neurohumoral vasoconstrictive systems (ie, renin-angiotensin-aldosterone system) leading to renal vasoconstriction, water and sodium retention. The excessive plasma volume results in ascites and, eventually, in acute kidney injury [3]. Portosystemic shunting, together with the worsening in liver function, contributes to hepatic encephalopathy by reducing the clearance of gut-derived toxins (i.e. ammonia). However, in the recent years, different interventions have reported that lowering portal pressure by treating the etiology of cirrhosis, using endovascular procedures and/or drug therapies is associated with improved overall prognosis.
The advances of different diagnostic tools and the design of high-quality trials for the treatment of portal hypertension and its complications have always been challenging. Consequently, since 1986 systematic consensus meetings, named Baveno, have been held to address these difficulties [4]. The aim of these meetings was to develop definitions of key events in portal hypertension and to review existing evidence. Consensus recommendations were mostly oriented to the management of varices, but recently it was expanded on other complications of cirrhosis as well. The latest Baveno VII workshop entitled “Renewing consensus in portal hypertension” was organized in 2021 and incorporated the latest advances in the field. The main areas of discussion were the importance and indications for measuring the HVPG as a gold standard for staging portal hypertension, use of non-invasive tests (NITs), impact of etiological and non-etiological therapies on the course of cirrhosis, prevention of liver decompensation episodes, management of acute bleeding events, and the management of different vascular disorders of the liver. In this review, we will concisely summarize the most significant concepts from Baveno VII regarding the use of NITs in advanced chronic liver disease (ACLD), analyze further decompensation events, and evaluate recent recommendations for prophylaxis and management of liver decompensation events.
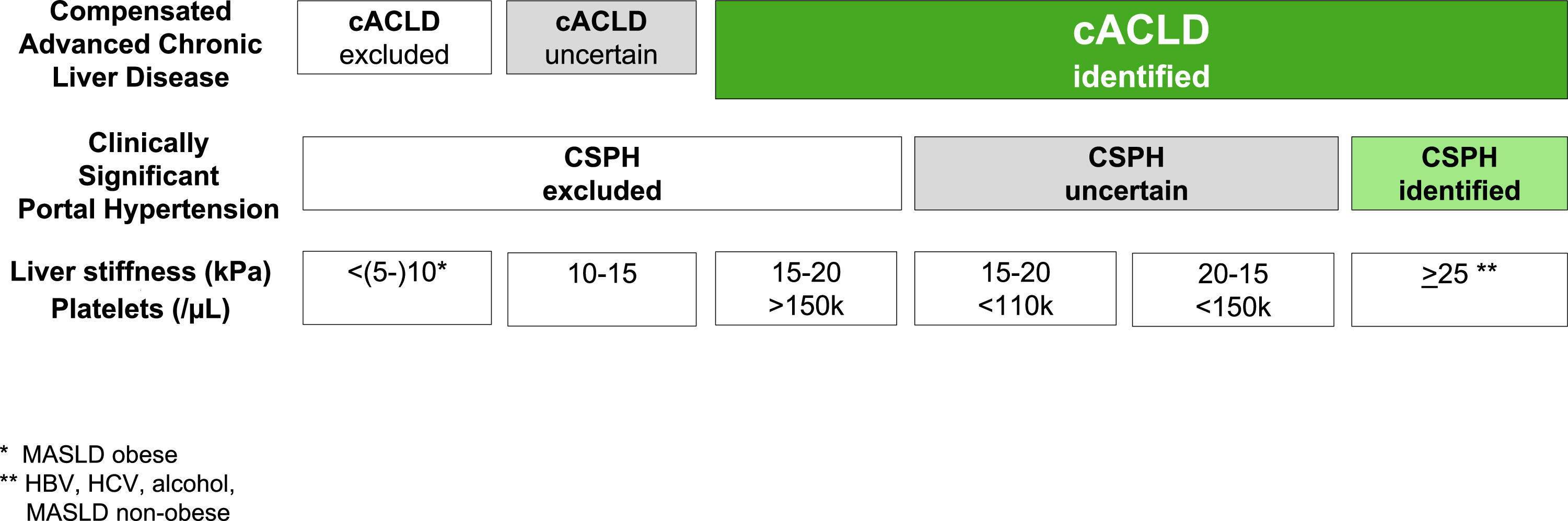
2The concept of compensated advanced liver diseaseThe Baveno VI consensus proposed the term ACLD for patients with late stages of chronic liver disease to replace the use of the term cirrhosis, which is a histology-driven concept [5]. Compensated ACLD (cACLD) better reflects the continuum spectrum from severe liver fibrosis to compensated cirrhosis as in asymptomatic patients, recognizing that a clear differentiation between the two is often not possible on daily practice. The Baveno VI consensus recommended the use of NITs for early diagnosis of advanced chronic liver disease. Later, the Baveno VII conference moved forward to a prognostic definition using liver stiffness measurements (LSM), particularly by transient elastography (TE), to precisely stratify the risk of CSPH and decompensation regardless of the histologic stage [4] (Table 1). Thus, LSM by TE values <10 kPa in the absence of typical imaging findings suggestive of cirrhosis exclude cACLD. LSM values between 10 and 15 kPa are suggestive of cACLD, whereas those >15 kPa are highly consistent with cACLD in all etiologies. The probability of CSPH is high when LSM is >25 kPa and low when LSM <15 kPa and platelet count ≥150 × 109/L, with a specificity and positive predictive value higher than 90 % in patients with alcohol- and/or virus-related cACLD and BMI <30 kg/m2 [4,6–8]. Consequently, the proposed rule-of-five for the cut-off of LSM by TE (10–15–20–25 kPa) combined with platelet count as recommended by Baveno VII consensus quickly estimates the risk of liver-related events and deaths, irrespective of the etiology (Fig. 1). However, there is a “gray zone” that involves those patients whose LSM ranges from 15 to 25 kPa in whom a positive diagnosis of CSPH requires the presence of other portal hypertension signs, such as low platelet count. Baveno VII consensus cut-offs were based on the ANTICIPATE model performed in patients with cACLD of any etiology, except metabolic associated steatotic liver disease (MASLD) with BMI <30 kg/m2[6]. In the latter study, TE values between 20 and 25 kPa or between 15 and 20 kPa and a platelet count <150 × 109/L or <110 × 109 /L, respectively, have a risk of CSPH of at least a 60 % [6]. Subsequently, the ANTICIPATE-non-alcoholic steatohepatitis model included more patients with MASLD and demonstrated that a LSM ≥25 kPa is sufficient to rule-in CSPH in most etiologies, including non-obese patients with MASLD, but not in obese patients with MASLD [7].
Baveno VII milestones.
LSM, liver stiffness measurement; TE, transient elastography; cACLD, compensated advanced chronic liver disease; CSPH, clinically significant portal hypertension; MASLD, metabolic associated liver disease; NIT, non-invasive test; NASH, non-alcoholic steatohepatitis; SSM, spleen stiffness measurement; NSBB, non-selective beta blocker; SBP, spontaneous bacterial peritonitis; HRS-AKI, hepatorenal syndrome-acute kidney injury; TIPS, transjugular intrahepatic portosystemic shunt; HVPG, hepatic vein pressure gradient, MASLD.
The justification for the use of rule-of-five appears from a comprehensive evaluation of recent evidence that showed that CLD patients with LSM <10 kPa have a negligible 3-year risk of liver-related events (LRE) of less than 1 %. The 3-year risk of LRE increases between five to ten times with LSM >15 kPa, regardless of ACLD etiology [9,10]. Patients who developed cACLD should be referred to a liver disease specialist for further work-up and monitoring. Careful monitoring of ongoing liver injury is recommended in individuals with LSM values between 7 and 10 kPa. In patients with cACLD, LSM may be repeated annually to monitor changes, and complemented with validated serum markers of fibrosis [11].
Baveno VII has also established the prognostic relevance of LSM-dynamics over time for LRE and deaths in patients with cACLD. The conference defined as significant any decrease in LSM to <10 kPa or of ≥20 % associated with LSM <20 kPa [4]. As supported by longitudinal studies reporting a minimal risk of LRE in patients with LSM <10 kPa on follow-up, while the risk of LRE remains high if LSM >20 kPa [12,13]. Subsequent evidence in patients with NAFLD has shown a significant reduction in the rate of LRE in individuals with a decrease of LSM compared to those with stable or increasing LSM (3.8% vs. 6.2% vs. 14.4 %; respectively) [13]. Besides, a recent study has shown that a 20 % increase (or decrease) in LSM at any time associated with a ∼50 % increased (or decreased) risk of hepatic decompensation or death [14]. The latter study further reinforces that any LSM-decrease to <20 kPa identifies cACLD patients with a substantially low risk of hepatic decompensation.
The risk of LRE and deaths can be significantly reduced after suppressing or removing the etiological factor of ACLD. Patients with hepatitis C virus (HCV) who achieved sustained virological response with direct-acting antiviral regimens present a significant reduction in the risk of all-cause mortality and LRE, independently of the presence or not of varices [15–17] Patients with alcoholic liver-related disease can potentially present a significant decrease in HPVG with a substantial reduction in LRE after alcohol withdrawal [18]. In patients with CSPH, the optimal percentage of HVPG decrease associated with a reduction in the risk of liver decompensation needs to be defined. Available information regarding the LSM variance after removal of the primary etiological factor does not allow to estimate changes in HVPG. A recent pooled analysis performed in patients with HCV and cACLD who achieved sustained virological response reported no risk of decompensation after 3-years in those with LSM values <12 kPa and platelet count >150,000/µL [19]. Thus, these patients may be discharged from further LSM and endoscopy surveillance [4]. In contrast, those individuals with LSM ≥20 kPa and/or platelets <150,000 /µL should continue with portal hypertension monitoring [4,7,20]. In any case, HCC surveillance should always continue.
3First and further liver decompensation eventsWhen established, cirrhosis remains compensated for a variably long time, depending on curability of the underlying liver disease. The first decompensation event in patients with cACLD represents a critical moment in the natural history of the disease. The appearance of overt ascites, overt hepatic encephalopathy or variceal bleeding implies the evolution from compensated to decompensated cirrhosis, leading to a dramatic increase in mortality risk [21,22]. The presence of variceal bleeding, ascites or hepatic encephalopathy are associated with a five-year mortality of 10–20 %, 50 % and 80 %, respectively [23,24]. Decompensation of cirrhosis can also be precipitated by different liver insults, such as infections, alcohol-related hepatitis, acute viral hepatitis (including HBV flares), drug-induced liver injury, hepatocellular carcinoma, and major surgery. Controversy exists regarding if jaundice should be considered a true first decompensation or it is just the consequence of superimposed acute liver injury. In the same line, there is insufficient data available regarding whether a minimal amount of ascites only detected in imaging procedures, minimal hepatic encephalopathy, and occult bleeding from portal hypertensive gastropathy can be considered as decompensation. After a decompensation event has occurred, treatments should be aimed to decrease the risk of mortality by preventing further decompensation and acute-on-chronic liver failure. Baveno VII consensus experts have stated that further decompensation should be considered as a new prognostic stage that carries a higher mortality than the one associated with the first decompensation event. The most frequent combination is variceal hemorrhage and ascites, with a 5-year mortality as high as 88 % [25].
Until recently, the definitions of “hepatic recompensation” and “clinical improvement” were heterogenous and used indistinctly. According to the Baveno VII consensus, hepatic recompensation basically implies a partial or complete regression of the functional and structural changes of cirrhosis after removal of the underlying etiology [26]. The Baveno VII consensus proposed the following criteria to define hepatic recompensation: i) persistent removal or suppression of the primary etiology of cirrhosis, ii) resolution of ascites and overt encephalopathy after treatment discontinuation and absence of variceal hemorrhage for more than 12 months, and iii) sustained improvement of liver synthetic function including albumin, international normalized ratio and bilirubin. Hepatic recompensation is presumably associated with an improvement of portal hypertension. As previously outlined, different studies have reported that removing the underlying etiology of cirrhosis can lead to a reduction in portal pressure [16,17]. However, these studies were mostly limited to patients with HCV-related cirrhosis who achieved sustained virological response after receiving direct-acting antivirals and presented no previous history of decompensation. Hence, these findings still need to be validated in patients who achieved hepatic recompensation. Moreover, it has yet to be evaluated whether the long-term prognosis between cACLD and recompensated patients is similar.
4Management of portal hypertension4.1Beta-blockers – carvedilol is better than propranolol?Non-selective beta-blockers (NSBBs) have been the standard of care for the primary and secondary prevention of variceal bleeding. The current standard of care based on Baveno VII consensus extends the recommendation of NSBBs to patients with compensated cirrhosis and CSPH, those at greatest risk of first clinical decompensation [4] (Table 2). The recommendation is largely based on the results of the PREDESCI trial, a randomized, placebo-controlled, multicentric trial in patients with compensated cirrhosis and CSPH [27]. This study found that NSBBs reduced the risk of first decompensation, namely ascites, by about 40 % after 2 years of treatment in patients with compensated cirrhosis and CSPH. However, in a post hoc analysis of the trial, the benefit of NSBB was primarily observed among patients with small varices, which constituted 56 % of the study population. Simplifying the findings, NSBB treatment was associated with risk differences of −20.1 % (95 % CI −34.7 to −4.3) in patients with small varices and −2.6 % (95 % CI −18.1 to −12.7) in patients without varices, raising the question of whether the window for beta-blockers opens when small varices develop [28]. Recently, a meta-analysis conducted with individual participant data provided further evidence supporting the effectiveness of carvedilol in preventing decompensation and improving survival in patients with compensated cirrhosis [29].
Medical management of portal hypertension.
The Baveno VII conference proposes carvedilol as the NSBB of choice to prevent first decompensation and first and recurrent bleeding in patients with cirrhosis [4]. Carvedilol combines β- and α1-adrenoceptor blocking activity and has demonstrated greater effectiveness in reducing HVPG in cirrhosis compared with propranolol. α1-receptors blocking activity confers to carvedilol an additional intrinsic vasodilatory activity and thus the ability to decrease the increased hepatic resistance, one of the main mechanisms leading to portal hypertension in cirrhosis [30]. Indeed, in the setting of primary prophylaxis carvedilol achieves a hemodynamic response in ∼75 % of patients, compared to ∼50 % under propranolol [31,32]. Carvedilol has been proven to have similar or superior efficacy to standard therapy (endoscopic band ligation or propranolol) in the prevention of first bleeding, whereas the evidence is less robust for rebleeding prevention, because comparative head-to-head studies with standard therapy are lacking [28,33]. On the other hand, compared with propranolol, carvedilol has a greater portal pressure-lowering effect leaving a lower number of non-responders and is easier to titrate. In consequence, like the prevention of first decompensation, carvedilol is the NSBB of choice to prevent first or recurrent bleeding, provided the patient does not have moderate-to severe ascites [34–36]. In the latter situation, the carvedilol α1-blocker activity might exacerbate peripheral vasodilation and lead to arterial hypotension, further contributing to increased renal sodium and water retention and worsening ascites control. In this context, carvedilol can be dose-reduced or discontinued, especially in case of persistent arterial hypotension (mean arterial pressure <65 mmHg or systolic arterial pressure <90 mmHg) and/or hepatorenal syndrome [4].
A word of caution is needed at the time of widespread the results of current evidence. First, in the PREDESCI trial the population at risk of first decompensation, i.e. CSPH, was identified by HVPG, which is not part of routine clinical care and consequently the applicability of these findings to real-world practice may be limited [27]. It is unclear whether the use LSM would yield similar results as HVPG assessment since the predictive value of TE to rule-in CSPH differs across etiologies [28]. Additionally, the 25 kPa cut-off to rule-in CSPH with a 91 % positive predictive value derives from a high prevalence population, of whom >90 % had portal hypertension and 59 % had CSPH and applying this cut-off in a low prevalence population would sharply decrease its positive predictive value [7]. Thus, relying on LSM to identify patients with CSPH who should receive NSBBs greatly increases the risk of overtreatment. Of note is also the population included in the PREDESCI trial, which mainly comprises patients with HCV from the pre-direct-acting antiviral era, whereas patients with other causes of liver disease are underrepresented. In this regard, it is important to highlight that the risk of decompensation in cirrhosis varies among the different etiologies, being 4–5-fold higher in alcohol-related than in metabolic associated fatty liver disease [37]. Therefore, further research is needed to determine the effectiveness and applicability of beta-blockers in other etiologies.
4.2Transjugular intrahepatic portosystemic shuntIn selected patients with cirrhosis, transjugular intrahepatic portosystemic shunt (TIPS) placement improves not only control of cirrhosis complications, such as variceal bleeding and ascites, but also improves survival [38]. The strategy of pre-emptive TIPS (pTIPS) has been further supported in the Baveno VII workshop in a meta-analysis of data from 1327 patients with cirrhosis and acute variceal bleeding Child-Pugh between 10 and 13 or Child-Pugh B with acute bleeding that showed that compared with drugs plus endoscopy pTIPS improved control of bleeding and ascites and increased the proportion who survived for 1 year, in both subgroups separately [39]. Interestingly, an observational multicentric study showed that pTIPS with covered stents is associated with better survival than endoscopic treatment in even among cirrhotic patients with high-risk variceal bleeding displaying hepatic encephalopathy at admission [40]. Recently, an individual patient data meta-analysis, comprising 12 studies which have assessed covered TIPS in comparison to standard of care in the indication of refractory ascites and prevention of variceal rebleeding, has shown that TIPS reduces the incidence of further decompensation (hazard ratio: 0.44; 95 % CI 0.37–0.54) and increases survival (two-year cumulative survival probability = 0.71 for TIPS vs. 0.63 for standard of care; p = 0.0001) [41].
5Non-etiological therapiesBaveno VII consensus has revised the potential impact of non-etiological therapies in the natural history of cirrhosis, specifically of anticoagulants and albumin.
5.1AnticoagulantsAccumulating evidence indicates that long-term anticoagulation might improve liver-related outcomes and overall survival in patients with cirrhosis. First, a seminal study indicated that preventing portal vein thrombosis (PVT) with low-molecular-weight heparin (LMWH) reduces hepatic decompensation and mortality, suggesting that anticoagulation may have advantages beyond portal vein recanalization [42]. Secondly, a retrospective, longitudinal study using national data showed that anticoagulation with vitamin K antagonists or direct-oral anticoagulants decreases all-cause mortality, hepatic decompensation, and PVT in patients with cirrhosis and atrial fibrillation [43]. Finally, anticoagulation with LMWH and/or vitamin K antagonists in patients with PVT improves overall survival by reducing liver-related mortality, as shown in an individual data meta-analysis of patients with cirrhosis and PVT [44]. The controversy surrounding the topic stems from the predominance of heterogenous observational studies with variations in definitions, inclusion criteria, study design, treatment timing, and follow-up durations, limiting direct comparisons.
Baveno VII consensus recommends anticoagulation in patients with cirrhosis who have recently experienced either complete or partial occlusion of the portal vein trunk, with or without extension to the superior mesenteric vein, symptomatic PVT, or PVT in individuals who are potential candidates for liver transplantation, regardless of the severity of occlusion. The anticoagulation should be maintained until portal vein recanalization or at least for 6 months and continued after recanalization in patients awaiting liver transplantation [4]. Direct-acting oral anticoagulants are considered safe and effective in patients with Child-Pugh A/B cirrhosis, but not indicated for those with Child-Pugh C. Patients with cirrhosis on anticoagulation face an increased risk of non-portal hypertensive bleeding, mainly from a gastrointestinal source, if prophylaxis variceal bleeding has not been undertaken before anticoagulation [43,44]. Subgroups of patients with ACLD in whom outcome of disease is likely to be impacted by anticoagulant therapy remains to be identified. In the meanwhile, PVT seems to identify a subset of patients with cirrhosis most likely to benefit of long-term anticoagulation [45].
5.2AlbuminA decline in serum albumin levels, as well as structural and functional molecule changes, are a characteristic features of cirrhosis progression and have been linked to increased mortality. Albumin therapy can have numerous significant biological effects due to its antioxidant, scavenging, and immunomodulatory non-oncotic properties [46]. In fact, long-term human albumin infusion has been shown to reduce liver related complications and probably improve transplant-free survival in patients with cirrhosis [47,48]. Several studies have revealed that short term albumin treatment lowers the incidence of infections, such as spontaneous bacterial peritonitis, as well as hepatic encephalopathy, hepatorenal syndrome, hyponatremia, hospitalizations, refractory ascites, and the need for paracentesis [49]. However, long term infusion of albumin is still a controversial issue. At least 3 studies have investigated this issue in decompensated patients: Pilot-PRECIOSA, ANSWER and MACHT. In Pilot-PRECIOSA, 18 patients were randomized to receive low doses (1 g/kg body weight every 2 weeks) and high doses (1.5 g/kg every week) of albumin for 12 weeks. Long-term high-dose albumin, but not low-dose albumin, was associated with normalization of serum level of albumin reduced systemic inflammation and cardiocirculatory dysfunction in patients with decompensated cirrhosis [50]. In the ANSWER trial, 440 patients with cirrhosis and uncomplicated ascites were randomized to SOC therapy versus albumin at 40 g twice a week for the first two weeks, and subsequently 40 g per week, for up to 18 months. Patients treated with albumin presented a significant higher survival and control of ascites, fewer decompensation events and hospitalization, and better quality of life, especially if they normalized serum albumin concentration at 1 month [46,51]. On the other hand, the MACHT study, a randomized, double-blind, placebo-controlled trial, assessed the long-term administration of midodrine and albumin 40 g every 15 days in 196 decompensated patients on the waiting list for liver transplantation and did not show any survival benefit [52]. Similarly, no benefits were observed in the ATTIRE trial, which assessed the use of repeated infusions of albumin to bring serum levels up to 30 g/L or more in 777 patients hospitalized for liver decompensation [53]. Taken together, these studies strongly suggest that the impact of administering albumin on the outcomes of patients with cirrhosis may depend on various factors, such as the specific indications for albumin usage, the baseline serum albumin level, the severity of liver disease, and the albumin infusion strategy.
The utilization of albumin is not exempt of potential adverse effects, primarily encompassing allergic and transfusion reactions, volume overload leading to pulmonary edema (especially in patients with NASH that frequently have associated cardiovascular disease), antibody formation, and disturbances in coagulation [54,55]. Taking into account factors such as their cost, limited availability, requirement for administration in a healthcare setting, and the potential for adverse effects, a formal recommendation on the use of long-term albumin infusions cannot be given until further data become available.
6Challenges to implement the Baveno VII recommendations in resource limited countriesThe Baveno VII consensus brought about a significant change in clinical hepatology by suggesting that treatment of CSPH with NSBB should be considered upon diagnosis, rather than waiting for the development of (high-risk) varices. The presence of CSPH should be investigated using NITs or surrogate markers, allowing for timely initiation of NSBB (preferably carvedilol) to prevent the first hepatic decompensation. Cut-off values were defined for the presence of CSPH, as well as for prognosis and risk stratification. This approach signifies a shift from the previous reliance on endoscopies and highlights the importance of early intervention and prevention in hepatology [4].
The widespread application of these recommendations in resource-limited countries is challenging due to the restricted access to transient elastography, which is generally not available outside referral centers. In this context, several centers are still relying on Baveno VI non-invasive criteria to stratify patients with varices, sparing endoscopies [5]. Endoscopy is easier to access and relatively inexpensive, reason why some Latin America centers still indicate it for risk stratification of targeted patients.
It is also important to highlight that NITs are not exempt from errors. The performance of NITs to exclude or rule-in CSPH varies between etiologies and elastography cutoffs are not validated for all of them. Patients within the diagnostic/prognostic ‘gray-zone’ of LSM 15–25 kPa may still had a relevant risk to develop first hepatic decompensation, especially in non-viral etiologies, leaving them untreated. On the other hand, Baveno VII criteria underscores the improvement in the underlying risk profile of the patient who achieves an etiological cure. Interestingly, to address these diagnostic gaps, new approaches have been introduced, such as deltas of LSM, spleen stiffness measurement or the ratio of von Willebrand factor and platelets (VITRO score) [11,56,57]. There is also a pressing need to enhance and refine NIT to minimize misclassification of patients with MASLD and obesity or with mixed etiologies.
Latin America also faces a great difficulty in implementing pTIPS strategy. One major obstacle is the disparity in healthcare infrastructure across the region. Some countries lack specialized medical centers equipped with advanced interventional radiology facilities necessary for the TIPS procedure. Moreover, limited access to trained medical professionals proficient in performing and managing TIPS also hinder the widespread adoption of this treatment option, especially considering the 72-hour interval for performing the procedure in the acute setting of high-risk variceal bleeding.
7ConclusionsThe Baveno VII consensus introduces a new strategy to utilize NSBB treatment for the prevention of initial hepatic decompensation in patients who have been diagnosed with CSPH. The implementation of the Baveno VII criteria has initiated a significant momentum to broaden the utilization of NITs in the clinical care of patients, solidifying their indispensable role, particularly in individuals with cACLD. In order to maximize risk stratification and effectively allocate treatment in the diverse diagnostic gray-zone outlined by the Baveno VII recommendations, there is a need for more studies. Based on Baveno VII recommendations, carvedilol is the beta-blocker of choice to be used in all patients with cACLD and CSPH, since it reduces liver decompensation and improves survival, especially in patients with varices.