Introduction and aim. Hepatic encephalopathy (HE), caused by hyperammonemia resulting from liver disease, is a spectrum of neuropsychiatric and motor disorders that can lead to death. Existing therapies are deficient and alternative treatments are needed. We have shown that gene therapy with a baculovirus vector containing the glutamine synthetase (Bac-GS) gene is efficient for reducing ammonia levels in an acute hyperammonemia rat model. However, the most common condition resulting from liver disease is chronic hyperammonemia. In this work, Bac-GS was evaluated in bile-duct ligated rats, a chronic liver disease model with hyperammonemia and some characteristics of Type C HE.
Material and methods. Bac-GS was tested for mediating GS over-expression in HeLa cells and H9C2 myotubes. For determining the utility of Bac-GS for the reduction of ammonia levels in a chronic hyperammonemia animal model, four groups of rats were treated: control, sham, ligated with Bac-GS and ligated with Bac-GFP. Baculoviruses were injected i.m. 18 days post-surgery. Blood was drawn 2, 3 and 4 weeks post-surgery and plasma ammonia concentrations were quantified.
Results. In protein lysates of cells and myotubes transduced with Bac-GS, a 44 kDa band corresponding to GS was detected. Significant results were obtained in the hyperammonemic bile-duct ligated rat model, as plasma ammonia was reduced to normal levels 3 days after treatment with Bac-GS. Furthermore, a transitory effect of Bac-GS was observed.
Conclusion. Our results show that gene therapy by delivering GS is a promising alternative for treatment of hyperammonemia in acute-on-chronic liver failure patients with HE.
Hepatic encephalopathy (HE) is a neuropsychiatric disorder that severely affects the life style of patients, who have a high risk of death. It is a common complication in acute and chronic liver failure.1 Cirrhotic patients frequently exhibit covert or overt HE, which is detected by clinical testing or through the appearance of symptoms, such as a lower attention span, decreased awareness, altered sleep rhythm, abnormal psychometric or neuropsychological tests, personality change, inappropriate or bizarre behavior, asterixis, dyspraxia, lethargy, somnolence, objectively disoriented to time and space and coma.2 The importance of covert or minimal HE (MEH), that is characteristic in individuals with psychomotor slow down and cognitive deficits, has grown. It has identified in the 59.1% of cirrhotic patients with grade 0 of encephalopathy (West-Craven HE criteria).3
The etiology of HE is complex, but in the nucleus of this pathology is the elevation of ammonia in blood circulation and brain,4 recognizing that factors other than ammonia are relevant. Recent evidence confirms this toxin is the main cause of HE. In acute-on-chronic liver failure (ACLF) patients, a strong correlation between hyperammonemia and the presence of HE has been demonstrated. There is a direct correlation between an increase in the level of ammonia and a higher risk of death. Moreover, a lower grade of HE is observed when ammonia levels decrease.5 Furthermore, in cirrhotic patients that had suffered episodes of overt HE, a fasting ammonia level 1.5 times above the normal value was associated with an increase in probability of having a new episode of overt HE; the annualized rate of HE-related hospitalizations augmented in the same way.6 Other study identified covert HE and elevated blood ammonia as factors associated with the onset of overt HE in liver cirrhosis patients.7 However, it is interesting to note that in a group of 26 patients with likely MHE, identified by the neuropsychometric Trail Making Test, only 15 of them showed altered concentrations of venous blood ammonia.3
Ammonia has a severe effect in the brain.8 Besides the perturbations of ammonia on astrocytes and neurons, this toxin also has a negative influence on other specialized cells in the body like stellate cells in the liver, skeletal muscle fibers and probably Purkinje cells.9–11
During HE manifestations in acute and chronic liver failure, several lines of evidence have shown a synergistic relation between ammonia and other toxins, like mercaptans, phenols and fatty acids, ions as manganese, pro-inflammatory cytokines, mainly Tumoral Necrosis Factor α (TNFa), IL-1 beta and IL-6, and the activation of microglia (revised in Butterworth, 2016).12
With the aim to reduce ammonia levels and decrease HE symptoms in patients, disaccharides (lactulose) and antibiotics (Rifaximin) is one of the most used therapies in the clinic. This approach causes undesirable secondary effects, it is often not effective and has elevated costs. Therefore, other treatment options are in development.2,13 Our research group was the first to demonstrate that baculovirus-delivery of the glutamine synthetase (GS) gene is a promising strategy for diminishing hyperammonemia.14 The establishment of the gene therapy strategy proposed here can avoid the need of clinical interventions with potential secondary effects and relapses that are common when traditional therapies for hyperammonemia and HE are used.
The Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is an insect-baculovirus with an important role as a vector.15 In addition to its safety and a wide tropism, one of the most attractive properties of baculovirus is its capacity of carrying several genes and regulatory elements in its large genome (~134 kbp), which is translated in the possibility of delivering and expressing multiple genes in the host, allowing applications such as reprogramming a specific cell-type into another one, or gene editing.16
Due to the high potential of using baculovirus as a vector for human treatment in clinical trials, several improvements have been made in production processes, such as large-scale production, concentration, purification, storage, quality control and preclinical studies to demonstrate safety of the baculovirus therapy and formulations.17,18
Bac-GS is a recombinant baculovirus carrying the GS gene. When it was evaluated in vitro or in an acute rat model of hyperammonemia, a significant decrease in the ammonia levels was found.14
In this work, treatment with Bac-GS was tested in bileduct ligated rats, which is a model of chronic liver failure, showing some aspects of Type C HE and hyperammonemia.19 The obtained results were outstanding. Treatment with Bac-GS was so effective in reducing the hyperammonemia in bile-duct ligated rats that ammonia levels reached normal values. They confirm that Bac-GS and delivery of the GS coding sequence as a promising gene therapy to control hyperammonemia in patients with HE.
Material and MethodsProduction of recombinant baculovirusThree recombinant baculovirus vectors were used, Bac-GS, Bac-control and Bac-GFP. Their construction is described in Torres-Vega, et al. (2015).14 Bac-GS contains the rat liver GS cDNA, Bac-control is identical to Bac-GS but does not contain the GS gene. Bac-GFP contains the enhanced green fluorescent protein gene in place of the GS gene. The amplification of the recombinant baculoviruses is described in Torres-Vega, et al. (2015).14 Titers of baculovirus stocks were determined as described in Mena, et al. (2003).20 Titers were 3 × 108, 2.4 × 108 and 6.6 × 108 pfu/mL for Bac-GS, Bac-control and Bac-GFP, respectively.
Transduction of mammalian cells with recombinant baculovirusesHeLa or H9C2 (ATCC, Manassas, VA, USA) cells were grown in Dulbecco’s modified Eagle’s medium high glucose (Gibco-Invitrogen, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco-Invitrogen) and penicillin/streptomycin (Gibco/Invitrogen). To induce the differentiation to myotubes, medium of a confluent L6 myoblast culture was replaced with medium containing only 1% FBS and incubation was continued for four weeks.21 To transduce cells with recombinant baculoviruses, the medium of six-well plates with confluent HeLa cells or H9C2 myotubes was replaced with 1.5 mL of Dulbecco’s modified Eagle’s medium without serum or antibiotics, and the baculovirus particles were added (100 pfu per cell). Plates were incubated for 1 h at 26°C with mild shaking every 10 min. Thereafter, medium was substituted with 2 mL of Dulbecco’s modified Eagle’s medium high glucose (Gibco-Invitrogen, Grand Island, NY, USA) containing antibiotics, 5 mM sodium butyrate (Sigma, St Louis, MO, USA) and 1% or 2% FBS (Gibco-Invitrogen) for myotubes H9C2 or HeLa cells, respectively. Cells were maintained for three days. Pictures of GFP fluorescence were taken in an Olympus microscope.
Western blot analysisWestern blot to detect Bac-GS-mediated GS expression was done as described in Torres-Vega, et al. (2015)14 using a rabbit anti-GS antibody (Sigma, St Louis, MO, USA).
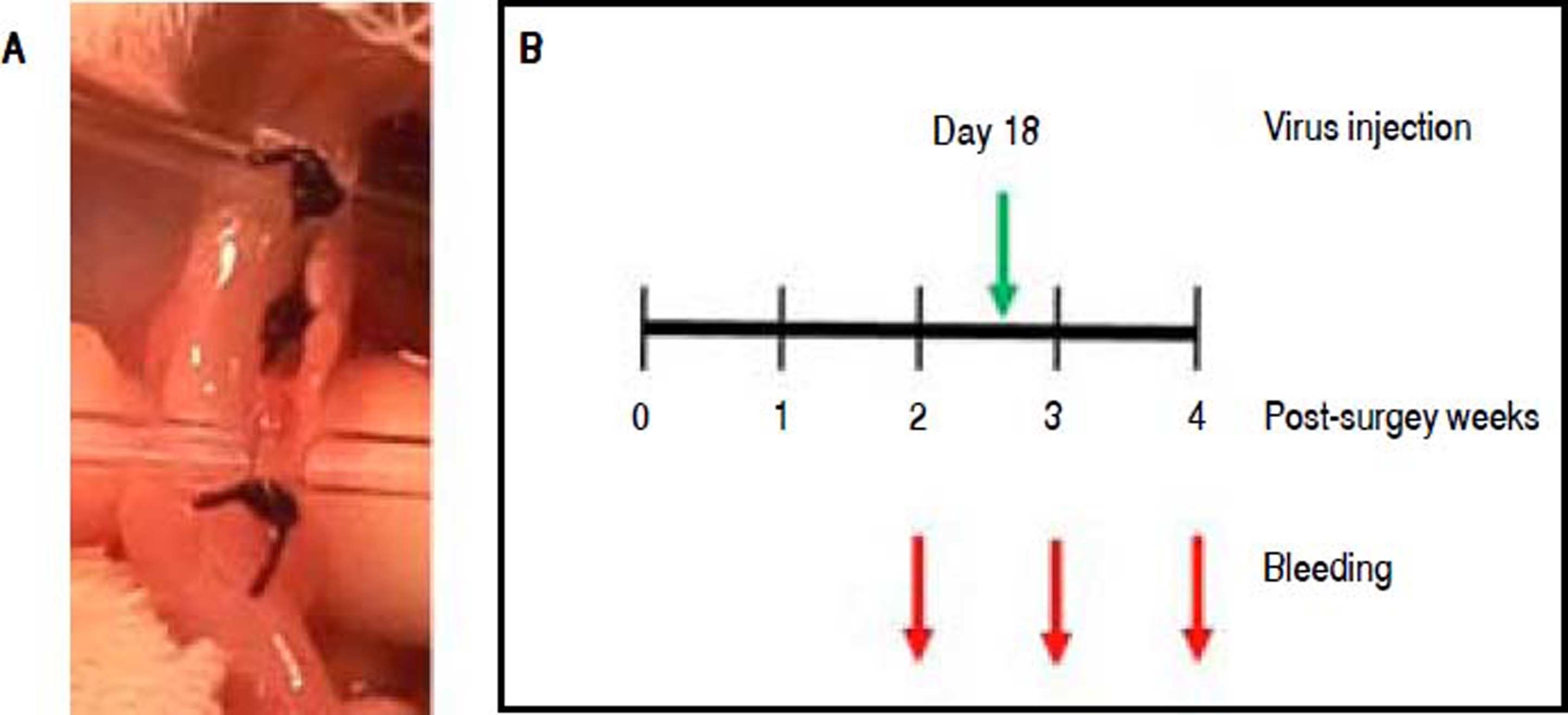
Bile-duct-ligation modelExperiments with rats were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. To ligate the bile-duct of rats, we followed the procedure described in Giménez-Garzó, et al.,22 with some modifications. Wistar male rats, 160 - 250 g body weight, were anesthetized intraperitoneally with a combination of Xylazine (Xilazina Aranda, Querétaro, México) and Ketamine (Anesket Pisa Agropecuaria, Atitalaquia, México). The abdominal part of the body was shaved and an incision in the linea alba was made to expose the liver. The liver lobules were separated and the bile-duct was localized. The connective tissue holding the bile-duct was broken with a capillary. Three ligations were made along the bile-duct using soft silk suture 4 0 (Ethicon, USA), as shown in Figure 2A Then, the muscle and the skin were sutured with soft silk suture 3 0 (Ethicon, USA). The total number of triple-ligated rats was 30. Twenty of them survived for five weeks, when all rats were euthanized. Sham rats had the same surgery with exception of the ligations. The administration of recombinant baculovirus to rats was performed as described in Torres-Vega, et al.14 Briefly, 0.9 or 2 × 108 pfu of Bac-GS or Bac-GFP baculovirus vectors, respectively, were intramuscularly injected into the two rear legs.
Ammonia quantificationPlasma samples were deproteinized has described in Jover, et al.23 Ammonia was quantified on the neutralized supernatants using a modified Kaplan method.24 Briefly, two solutions were prepared: A) 106 mM phenol (Merck, Darmstadt, Germany), 0.17 mM sodium nitroprusside (Merck, Darmstadt, Germany); B) 0.125 N sodium hydroxide (J.T. Baker, Xalostoc, México), 11 mM sodium hypochlorite (Sigma, St Louis, MO, USA). Twelve and a half microliters of supernatants were mixed with 0.5 ml solution A; 0.5 ml of solution B were added and mixed. Color was development after 30 min. Then, to quantify ammonia two hundred microliters of the complete mix were loaded into 96-well plates and absorbance was measured at 635 nm on an Epoch 2 BioTek microplate reader.
Statistical analysisFor descriptive statistics of quantitative variables, the mean ± standard deviation was used. Analysis of data was made with the analysis of variance - Tukey test using the GraphPad Prism 7 software. P < 0.05 was considered statistically significant. GraphPad Prism 6.01 software was used to plot data.
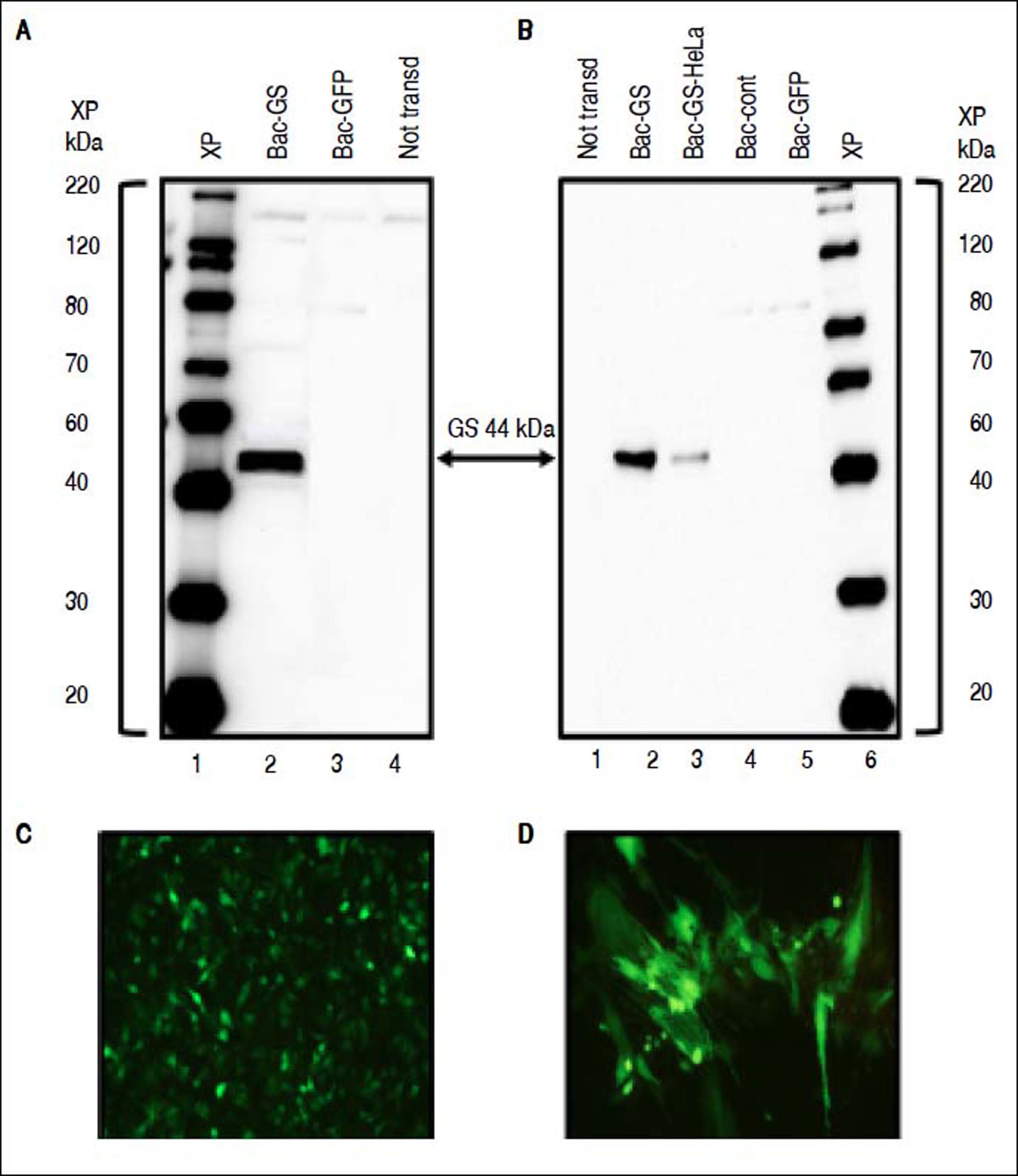
ResultsExpression of GS in HeLa cells and H2C9 myotubesWith the aim of evaluating a wider range of cell substrates for GS gene delivery by baculovirus, HeLa cells were transduced. Condreay, et al.25 reported that HeLa cells are efficiently transduced by baculovirus vectors. Similarly to MA104 epithelial monkey cells, which we evaluated as an hyperammonemia in vitro model in our previous publication,14 Western blotting of HeLa cells transduced with Bac-GS showed a specific sharp band with a molecular weight of 44 kDa, which corresponds to the size of GS (Figures 1A and 1B). HeLa cells were also efficiently transduced by Bac-GFP (Figure 1C). To evaluate the ability of Bac-GS to transduce skeletal muscle cells, the target of the proposed gene therapy for hyperammonemia, H9C2 cells, myoblasts derived from the heart of rat embryos,21 were differentiated into myotubes by culturing them with a low FBS concentration (1%), resulting in the morphology typical of skeletal muscle myofibers.21 The morphology and the capacity of Bac-GFP to transduce muscle cells were evident upon observation under a microscope (Figure 1D). Differentiated H9C2 myotubes were efficiently transduced by Bac-GS and overexpressed GS with the expected molecular weight (Figure 1B).
Expression of the therapeutic GS gene in HeLa and H9C2 myotubes by transduction with Bac-GS. Cells were transduced with 100 pfu/cell. Protein lysates (4 μg) were analyzed by Western blot with a specific anti-GS antibody. A. HeLa cells were transduced with Bac-GS (Lane 2), Bac-GFP (Lane 3), or not transduced (not transd, Lane 4). XP: XP molecular weight marker. B. H9C2 myotubes were transduced with Bac-GS (Lane 2), Bac-control (Bac-cont, Lane 4), Bac-GFP (Lane 5), or not transduced (Lane 1). A sample of the HeLa-Bac-GS lysate was loaded as a positive control in Lane 3. C. Living fluorescent imaging of HeLa cells trasduced with Bac-GFP (20X). d) Living fluorescent imaging of H9C2 myotubes transduced with Bac-GFP (20X).
Establishment of the bile-duct ligaton model for testing Bac-GS as a therapy to reduce hyperammonemia. A. Picture of one of the ligated bile-ducts showing the three ligations. B. Schedule of bleedings and i.m. injection of recombinant baculoviruses, with reference with the bile-duct ligation surgery.
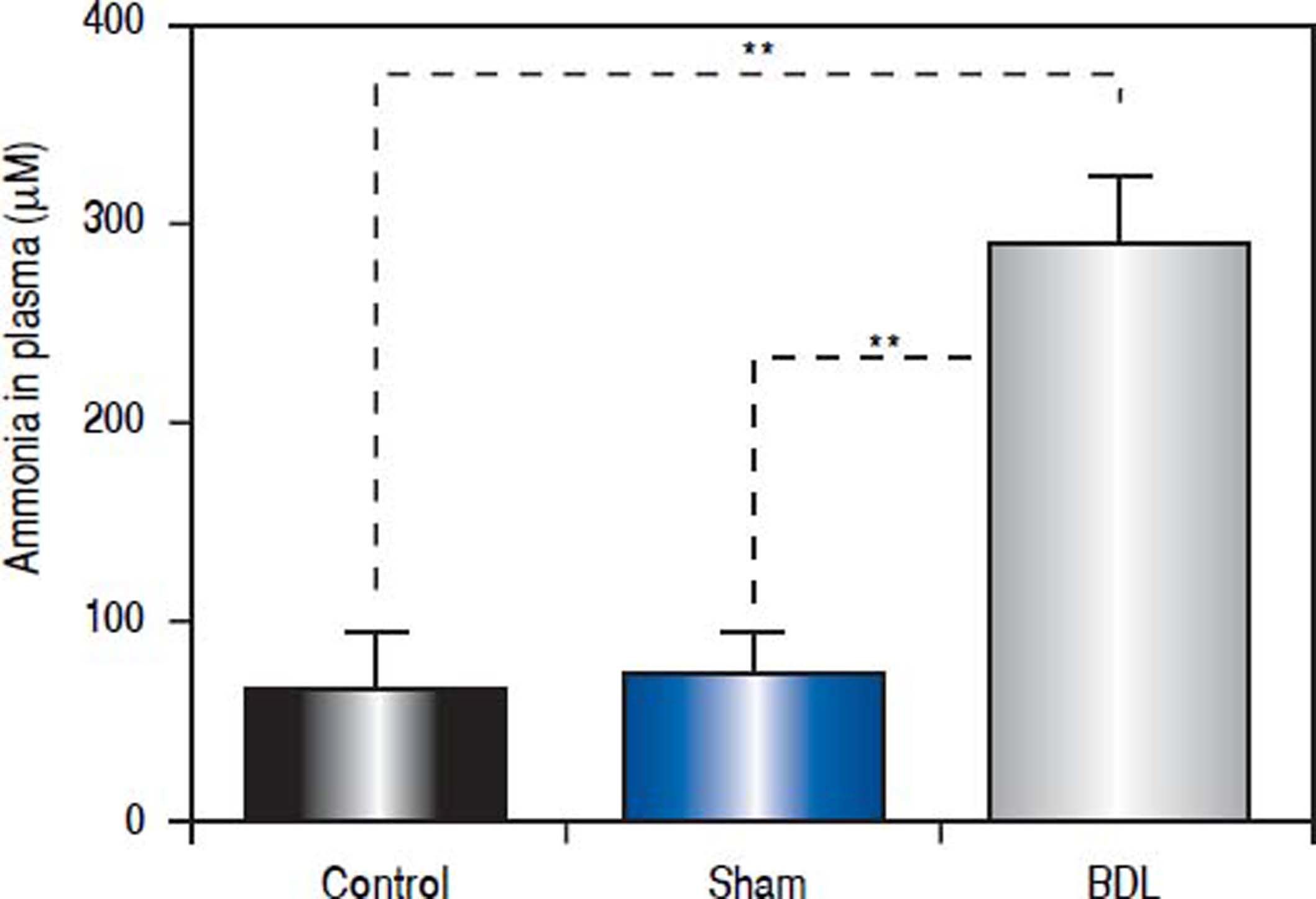
Ligation of the bile-duct in rodent results in jaundice, inflammation, fibrosis, liver damage and hyperammonemia.22,26 For these and other reasons, this procedure is considered a good chronic liver failure model, that shows some aspects of Type C HE.19 We intended to reproduce this model in our laboratory and practiced a surgery with a single or triple ligation in the bile-duct of rats (Figure 2A). Even when an increase of ammonia levels in circulating blood of rats with one ligation was observed, it was not significant (data not shown). In contrast, the rats with a triple ligation were hyperammonemic two weeks post-surgery. We selected rats with ammonia circulation levels above 200 μm (n = 12). These animals had four times more ammonia in their plasma (291.9 ± 35.3 μM) than control (65.1 ± 28.1 μM, p < 0.0001) or sham (71.4 ± 22.0 ¿M, p < 0.0001) rats (Figure 3).
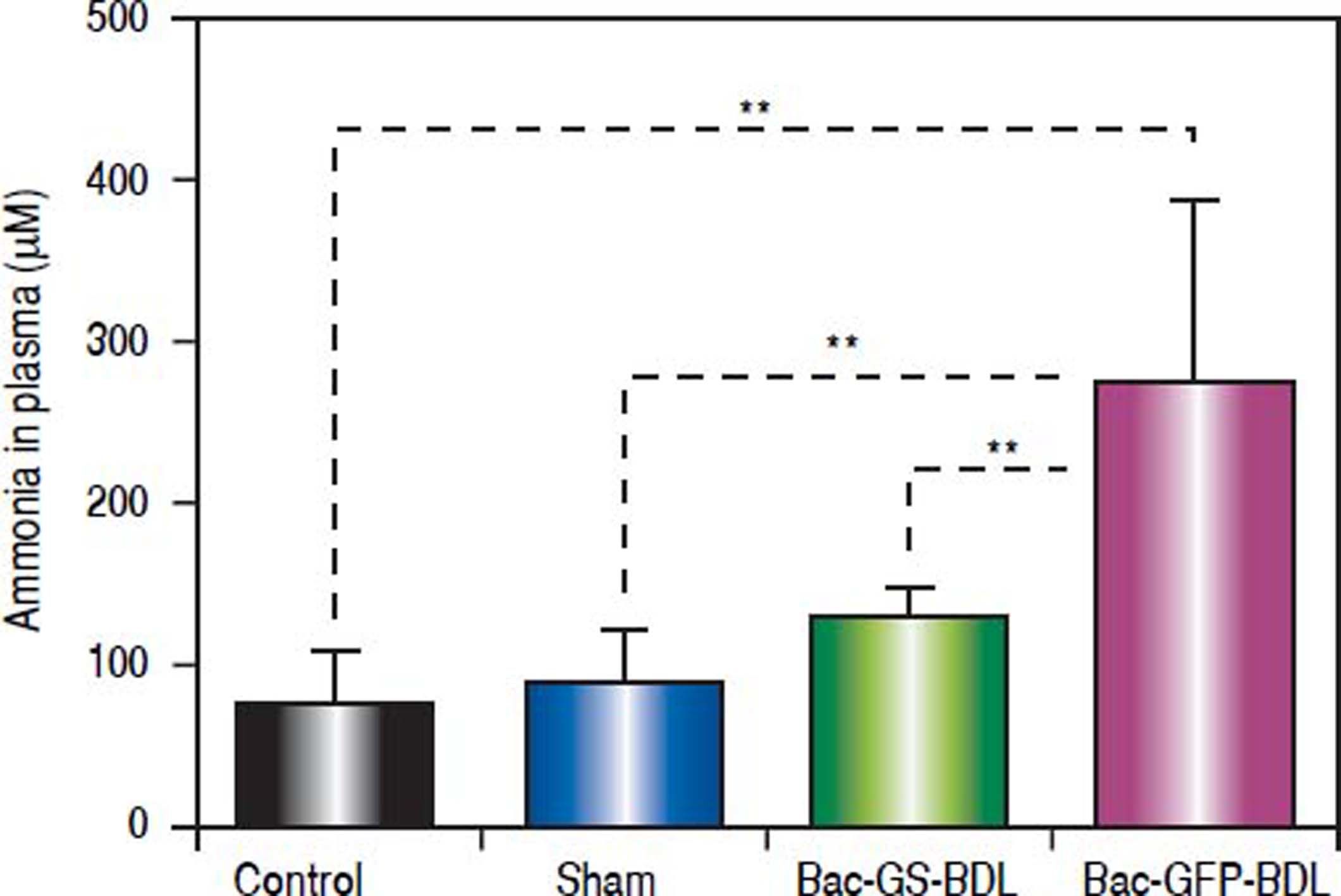
Reduction of high levels of ammonia in the bile-duct ligated rat model by Bac-GS administrationOnce we have stablished the bile-duct ligation (BDL) model, the gene transfer therapy with the recombinant baculovirus Bac-GS was tested. Hyperammonemic rats (> 200 μM, n = 12) were randomly divided in two groups: one was i.m. injected with Bac-GS (n = 7), while the other received a control baculovirus, i.e., Bac-GFP (n = 5), at 18 post-surgery days. Three days later, blood samples were taken from the tail artery and ammonia was quantified in the plasma (Figure 2B). Like in the acute hyperammonemia model reported before,14 we found low levels of ammonia in bile-duct ligated rats treated with Bac-GS (128.3 ± 19.0 ¿M, Figure 4). In contrast, ligated rats injected with Bac-GFP remained hyperammonemic (276.7 ± 113.1 μM, p < 0.0001, Figure 4). Control and sham rats had low ammonia concentrations in plasma, 72.6 ± 34.2 μM and 84.8 ± 35.3 ¿M, respectively. There was not significant difference between the control, sham and Bac-GS treated rats (Figure 4).
Gene therapy with recombinant baculovirus Bac-GS decreased hyperammonemia in a chronic liver disease model. Hyperammonemic bileduct ligated (BDL) rats were divided in two groups and were i.m. injected with recombinant baculoviruses at 18 post-surgery days. One group (n = 7) received Bac-GS (9 × 107 pfu), Bac - GS + BDL, and the other (n = 5) received Bac - GFP (2 × 108 pfu, Bac-GFP+BDL. Control and sham rats were also monitored. Rats were bled from the tail 3 weeks post-surgery. Ammonia was quantified in plasma. Control, n = 8; sham, n = 12. ** P < 0.0001.
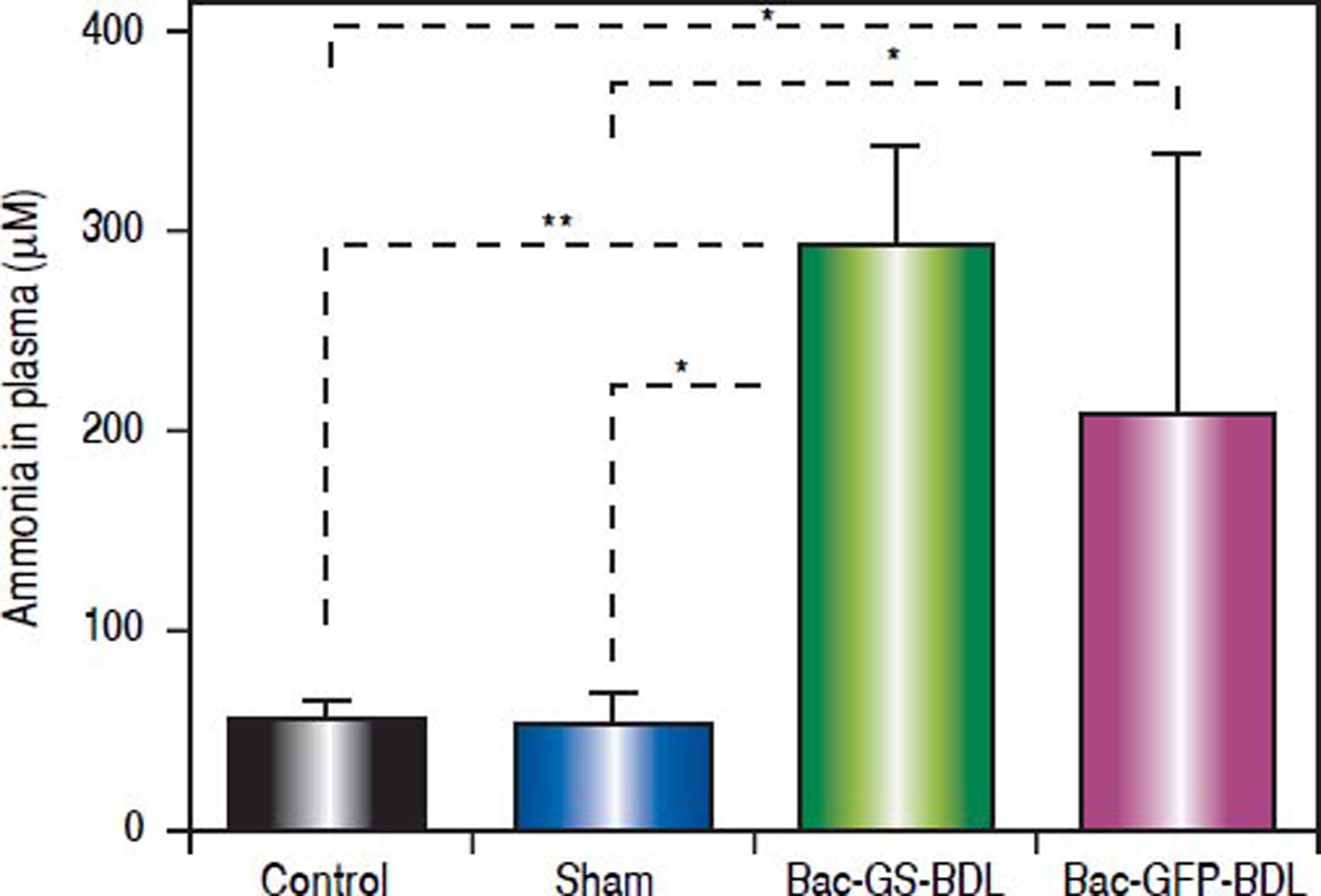
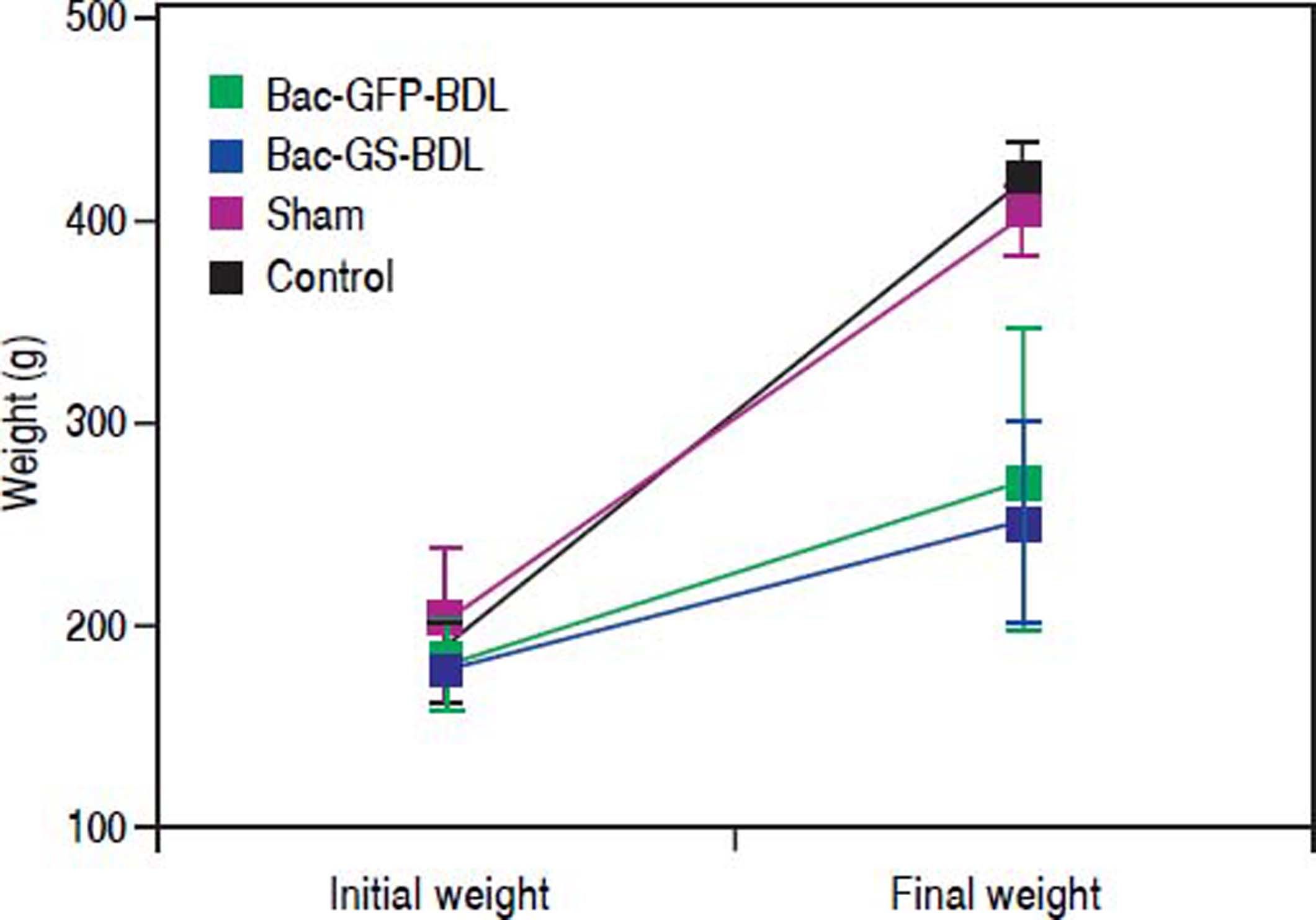
It has been reported that baculovirus mediated-expression of transgenes in mammalian cells only lasts from one week to few months.27,28 We wanted to know if baculovirus mediated GS transduction was sustained over time. Blood samples were taken four weeks post-surgery, ten days after the i.m. injection of Bac-GS (Figure 2B). In comparison with control (52.7 ± 11.5 μM, p < 0.0001) and sham rats (52.1 ± 16.1 μM, p < 0.001), the bile-duct-ligated rats treated with Bac-GS were hyperammonemic (291.7 ± 51.1 μM, Figure 5). Ammonia concentration in plasma was similar to that obtained two weeks post-surgery (Figure 3). The amount of ammonia in the plasma of bile-ductligated rats injected with Bac-GFP at four post-surgery weeks (206.7 ± 132.9 μΜ) was not significantly different from the level found in rats receiving Bac-GS (Figure 5). Furthermore, there were differences in body weight accumulation between control, sham and bile-duct ligated rats, as shown in figure 6. Control and sham rats had similar weight gain (final weight, 410.9 ± 19.8 and 393.5 ± 16.0, respectively, p > 0.05), but weight gain in ligated rats was significantly lower (P < 0.0001). The lower final weight in both Bac-GS and Bac-GFP injected bile-duct rats was similar (final weight, 247.3 ± 48.1 and 267.2 ± 75.3 g, respectively, p > 0.05).
Recovery of hyperammonemia in bile-duct ligated (BDL) rats treated with Bac-GS. One group of hyperammonemic BDL rats received Bac-GS (9 × 107 pfu, Bac - GS + BDL, n = 7) and the other one received Bac-GFP (2 × 108 pfu, Bac - GFP + BDL, n = 5) at 18 days post-surgery. Rats were bled from the tail 4 post-surgery weeks. Ammonia was quantified in plasma. Control, n = 8; sham, n = 12. * P < 0.001; ** P < 0.0001.
Changes in body weight of bile-duct ligated rats. One of the effects of bile-duct ligation (BDL) is translated to the growing or weight gain in the rats. At the time of the surgery all rats belonged to the same population and weight (Initial weight), but five weeks post-surgery (Final weight), the ligated rats significant weighted less in comparison with sham or control rats (p < 0.0001). There were no significant differences in the weight between the two groups of ligated rats that were injected with Bac-GS or Bac-GFP (p > 0.05).
Baculovirus is a versatile vector capable of transducing a variety of mammalian cells in vitro or in vivo. We have assessed the capacity of baculovirus vectors (Bac-GS and Bac-GFP) for transducing MA104 or HeLa cells (Torres-Vega, et al., 2015;14 this work). GFP fluorescence or overexpression of the GS gene were detected in these cell lines. Furthermore, a significant reduction of ammonia levels (44%) after transduction with the baculovirus Bac-GS was observed in the in vitro hyperammonemia model established in MA104 cells.14 In addition, it was shown that baculovirus vectors can efficiently transduce muscle cells (myoblast/myotubes L6 or H9C2; Torres-Vega, et al., 2015;14 this work), setting ground for the use of the large muscle volume for the expression of missing or needed therapeutic activities. Moreover, the ability of baculovirus to transduce several types of cells can be used to identify other factors or proteins that may act along the GS activity in diminishing ammonia, and to translate the findings to an improved gene therapy for hyperammonemia. Other applications where a reduction in ammonia concentration is needed, such as the development of bioartificial livers, can also benefit from the results reported here.29–31
Baculovirus mediated over-expression of GS in cells and myotubes was strong and it gave us confidence for testing the recombinant baculovirus for treatment of the main cause of HE, i.e., hyperammonemia. Previously we reported the i.m. administration of Bac-GS in rats with acute hyperammonemia, provoked by the i.p. injection of ammonium acetate, which resulted in a 351 μΜ decrease of plasma ammonia. This ammonia reduction was attributed to the over-expression of GS in skeletal muscle.14 Here, we tested Bac-GS gene therapy in an animal model for chronic hyperammonemia.
It has been proposed that an animal model of HE caused by chronic liver impairment (Type B and C HE) should include chronic liver disease with portal-systemic shunting, to show all the spectrum of HE symptoms including coma, the conversion of astrocytes in Alzheimer Type II, an elevated ammonia level in blood and brain, at least one precipitating factor, and the capability to give a positive response when the treatments used in the clinic are applied.19 However, there is no ideal animal model that reproduces all the pathologies showed in the cirrhosis-derived HE (type C). The bile-duct ligation model in rats exhibits most pathologies, including an elevation of ammonia in blood circulation for several weeks.19,32 We consider this is a satisfactory model for testing the gene therapy approach with Bac-GS, because the elevation of ammonia is in the context of other affections that could attack to the patients with liver failure.
We implemented the bile-duct ligation rat model in our laboratory, resulting in rats with ammonia levels above 200 μΜ, in comparison with less than 100 μΜ in control rats. Gene therapy with Bac-GS in hyperammonemic rats was effective, as plasma ammonia levels diminished from 292 ± 35 μΜ to 128 ± 19 μΜ (Figure 4), a reduction of 56.2 %, at the third day after Bac-GS injection to the muscle. No significant difference between the ammonia plasma concentration in control and test animals was found (Figure 4). Therefore, Bac-GS treatment resulted in a decrease of ammonia concentration to normal levels. Worthy, rats treated with baculovirus did not exhibit any abnormal behavior or corporal affectation during the days of study.
This result is relevant because it confirms our previous observation that recombinant Bac-GS can deliver the GS gene, and mediate the decrease of hyperammonemia. The feasibility of ammonia reduction driven by Bac-GS has been demonstrated in three different models: one in vitro using ΜΑ104 cells, and two in vivo, the acute hyperammonemia model and the bile-duct ligation model that represents a chronic liver disease like cirrhosis (Torres-Vega, et al., 2015;14 this work). Reduction of hyperammonemia in the chronic model was observed at three days after baculovirus injection, but not at 10 days after virus injection.
This is not surprising, as it has been reported that expression of transgenes delivered by baculovirus is of short duration because of the activation of the adaptive immune response. Particularly interesting is the development of neutralizing antibodies and CD4+ y CD8+ T-cells against gp64 baculovirus capsid protein.28 Accordingly, Bac-GS is a genetic tool that can decrease hyperammonemia in acute liver failure or in episodes of acute on chronic liver failure, where the presence of hyperammonemia is not sustained. Interestingly, Lammers, et al. found that the disposal of glutamine-bound ammonia to urea is dependent on the generation of new synthetized glutamine.33 This suggests that treatment with Bac-GS of acute and chronic hyperammonemic rats not only decreased the elevated blood ammonia levels directly by the activity of GS, i.e. by binding ammonia to glutamate, but probably also by stimulating the urea cycle.
To address the necessity of a prolonged expression of the GS therapeutic gene in liver failure patients with chronic hyperammonemia, we are already developing adeno-associated virus vectors (AAVs). These recombinant AAVs kept the expression of erythropoietin for six years after just one-time injection of the vector in the muscle of macaques.34
Glutamine synthetase catalyzes the ATP-dependent glutamine synthesis from glutamate and ammonia.35 GS is essential for life and normal development, as was evidenced in three patients with mutations in the GLUL gene, that generated structural changes and a decreased activity in GS. These patients showed neonatal onset severe epileptic encephalopathy, glutamine deficiency, hyperammonemia and brain malformations. They died one-two days to six years after birth.36 The main enzyme responsible for ammonia detoxification is GS, as was demonstrated by using a liver-specific GS knockout mouse (GS-KO/L), showing that enteral ammonia was cleared in 35% by GS in pericentral hepatocytes, 35% by the urea cycle enzymes and the rest was cleared probably by GS in skeletal muscle.35 These mice were hyperammonemic and showed low glutamine levels. Furthermore, it was identified that GS is solely responsible for systemic ammonia detoxification and there was a dependence of glutamine-bound ammonia disposal to urea (via mitochondrial glutaminase and carbamoylphosphate synthetase) on the rate of glutamine synthesis. The whole-body muscle-to-fat volume ratio declined > 5-fold in GS-KO/L mice compared to controls.33
Delivery of the GS gene to skeletal muscle as done here can be helpful in other conditions where ammonia accumulates and glutamine is lacking. Also, treatment with GS could reduce muscle loss in cirrhotics or in sarcopenia37 and for urea-cycle disorders. An important consideration is that there is no evidence of a specific muscle tropism of baculovirus. It is very probable that other cells types in the body can also be transduced.
In conclusion, the strategy of delivering the GS gene in vitro or in skeletal muscle of rats with acute or chronic hyperammonemia through the administration of Bac-GS is a promising strategy to alleviate elevated ammonia levels in liver failure patients. The consolidation of this type of gene therapy could reduce the necessity for liver transplantation, increase the survival and improve the quality of life of hyperammonemia-dependent HE and urea cycle patients.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
AcMNPV:Autographa californica multiple nucleopoly-hedrovirus.
- •
ATP: adenosine 5’-triphosphate.
- •
Bac-control: identical to Bac-GS but does not contain the GS gene.
- •
Bac-GFP: baculovirus vector containing the GFP gene.
- •
Bac-GS: baculovirus vector containing the GS gene.
- •
BDL: bile-duct ligation.
- •
cDNA: complementary DNA.
- •
FBS: fetal bovine serum.
- •
GABA: γ-aminobutyric acid.
- •
GFP: green fluorescent protein.
- •
GS: glutamine synthetase.
- •
GS-KO/L: liver-specific GS knockout mouse.
- •
HE: hepatic encephalopathy.
- •
i.m.: intramuscular.
- •
i.p.: intraperitoneal.
- •
IL: interleukin.
- •
MARS: molecular adsorbent recirculating system.
- •
MPT: mitochondrial permeability transition.
- •
NMDA: N-methyl-D-aspartate.
- •
Pfu: plaque forming unit.
- •
RNAi: RNA interference.
- •
TNFa: tumoral necrosis factor α.
The authors declares that there is no conflict of interest regarding the publication of this article.
FundingThis work was partially financed by the Programa UNAM-DGAPA-PAPIIT (IT-200315 and IN223210).
AcknowledgementsWe would like to thank CONACyT-México for giving a scholarship to Laura Sevilla during her graduate studies.