Biliary mucinous cystadenomas (BMC) of the liver are rare benign cystic tumors, however an estimated 20% undergo malignant transformation. They have recently been redefined as mucinous cystic neoplasms in the 2010 WHO classification. The preferred treatment is through radical resection, as there are high recurrence rates with other treatment modalities; however this is often not possible in patients with bilobar or giant cysts, and liver transplantation may be indicated. We present a patient with a giant biliary mucinous cystadenoma of the liver and discuss the management with reference to the literature. A 47 year-old woman presented with a 6-week history of moderate epigastric discomfort on a background of 12 months of symptom-free abdominal distension. A giant cystic bilobar tumor of the liver measuring 22 × 23 × 17 cm was diagnosed and characterised by ultrasound scan and magnetic resonance imaging. Serum bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase were elevated, though other laboratory data including tumor markers (CEA, aFP, CA19-9) were within normal limits. Total excision of the cyst was not possible due to its size and position, and the patient underwent cyst drainage, a sub-total cyst excision and omentoplasty. Histology confirmed a benign biliary mucinous cystadenoma with an ovarian stroma. Though the patient remained clinically well, routine post-operative computed tomography (CT) surveillance showed an 11 cm recurrent cyst at 6 months. A partial cyst resection with close follow-up, regular CA19-9 serology and ultrasound/CT imaging, may be a reasonable alternative for bilobar or giant cysts. However should any features pathognomonic of malignancy develop, then a liver transplantation is indicated.
Biliary mucinous cystadenomas (BMC) of the liver are rare slow-growing benign cystic tumors that usually involve the intra-hepatic ducts. On occasion these cysts can rapidly increase in size due to an accumulation of fluid.1 Though they are often asymptomatic, they can present as a palpable mass with abdominal pain and jaundice.2,3 The preferred treatment is through radical resection, as there are high recurrence rates with other treatment modalities, and a small risk of malignant transformation over the longer term. However complete excision is not always possible in patients with bilobar or giant cysts, and these patients may eventually require liver transplantation.
We present a patient with a giant bilobar BMC of the liver with ovarian-like stroma, and discuss the management with reference to the literature.
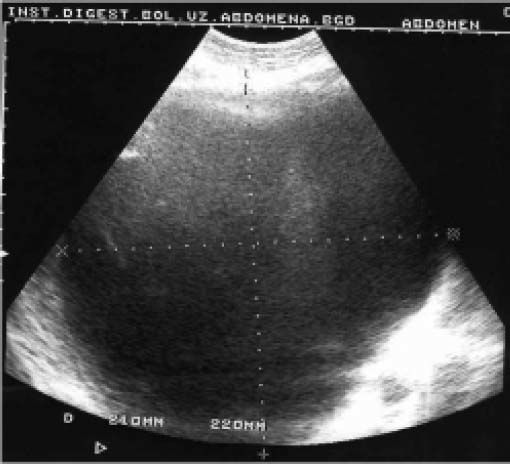
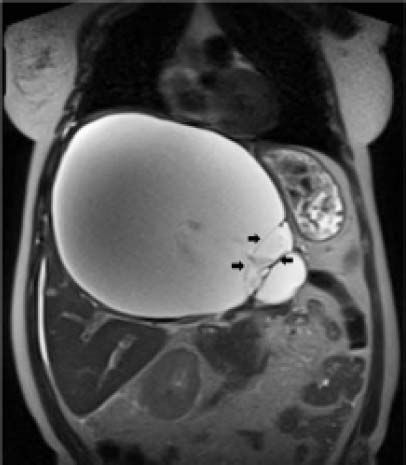
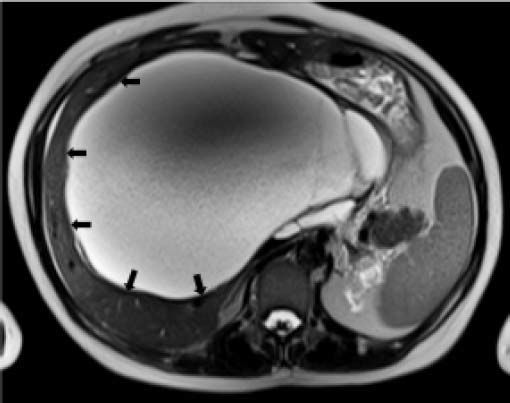
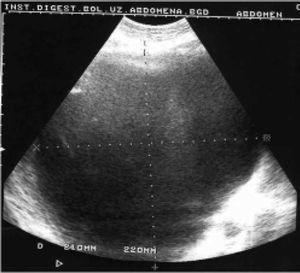
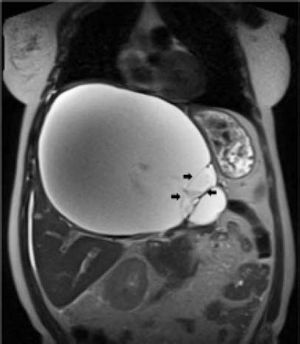
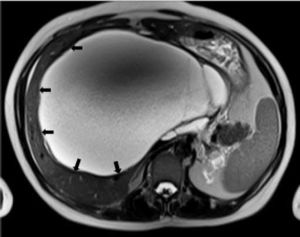
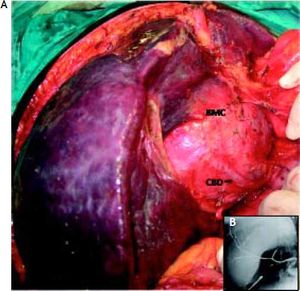
Case ReportIn July 2008 a 47 year-old woman presented with a 6 week history of moderate abdominal discomfort on a background of 12 months of increased abdominal swelling. On examination she was found to have an enlarged and slightly tender abdomen with a smooth-surfaced mass in the epigastrium extending down to the umbilicus. Abdominal ultrasound (US) confirmed a 22 × 23 × 17 cm cystic mass in the liver with a large quantity of associated free fluid in the lower abdomen (Figure 1). Serum levels of bilirubin (23.7 umol/L), alkaline phosphatase (319 U/L) and gamma-glutamyl transpeptidase (GGT) (1,022 U/L) were elevated, though other laboratory data including tumor markers (CEA, aFP, CA 19-9) were within normal limits. Magnetic resonance imaging (MRI) showed that the cystic mass in the centre of the liver spanned both lobes and was well-circumscribed, partly-septated and with a contrast-enhancing capsule (Figures 2–3). The lesion was also compressing the surrounding organs and the right hemidiaphragm was markedly elevated on chest X-ray.
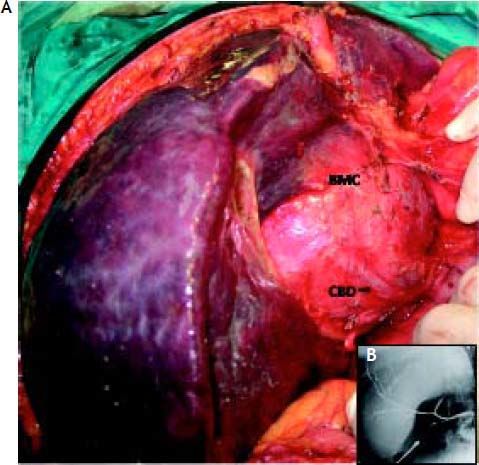
The patient underwent a right subcostal laparotomy and the abdomen was found to contain several litres of clear yellowish ascitic fluid. The huge cystic mass was approached from below the left liver lobe, where it was most superficial. There were many prominent and stretched overlying veins (Figure 4). The 5–6 mm thick cyst capsule was punctured and around 3 litres of clear mucinous fluid were aspirated. The cyst wall was then opened and the free surface was resected and sent for frozen section. There were a number of internal septae within the cyst. Unfortunately due to the central bilobar location a total cyst resection was not possible, and the cavity was drained and partly filled with an omental flap. The postoperative recovery was uneventful.
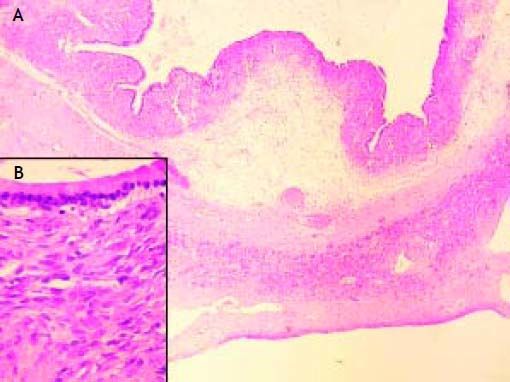
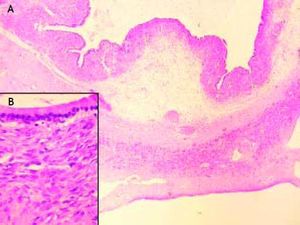
Histology of the resected specimen confirmed a biliary mucinous cystadenoma of the liver (BMC) with ovarian stroma, and no evidence of malignant transformation (Figure 5). Immunostains for MUC1, MUC2, CDX2 and Cytokeratin 7 were not available to us, but would have assisted in confirming the tumor phenotype.
Six months post surgery the patient remains clinically well, however a follow-up Computed Tomography (CT) scan has shown a recurrent 11cm cyst partly filled with the omental flap. The patient was not available for further review as she had emigrated.
DiscussionBMCs of the liver are rare, accounting for less than 5% of all bile duct tumors,1.–4 and have an estimated prevalence one thousand times lower than that of simple liver cysts.5 BMC is generally a disease of middle-aged women with 80–90 % of cases occuring at a mean age of 50 years.1,6 The malignant form tends to occur in women 10 years older.7
BMC is considered by most to be a congenital lesion; though its origins are still debated, and some authors have favored an aquired etiology such as a reactive process to a focal injury.4,8,9 Those that support a congenital aetiology have suggested that it may result from an obstruction of aberrant intrahepatic biliary ducts or derive from ectopic germ cells in the liver.5 The speculated origins of these germ cells includes the embryonic foregut,10 the ovaries and the gallbladder.4 The tumors have a very similar appearance to mucinous cystadenomas of the pancreas, and on occasion have co-existed, suggesting another possible common origin.11 Reflecting these observations BMCs were recently redefined as mucinous cystic neoplasms, in the 2010 World Health Organization (WHO) classification.12
BMC occurs wholy within the liver in 80% of cases, and extends into the extrahepatic bile ducts in the remaining 20%.13 The tumor occurs in both lobes with an equal frequency (40%) and is bilobar in 15–20% of cases.3,14,15 It usually exists as a solitary multiloculated lesion 3 and multicystic cases are rare.16 The tumors vary in size, with diameters ranging from 0.8–30 cm,3,4,17,18 though most occur in the 5–10 cm range. Fewer than 150 cases of the benign form and 50 cases of the malignant variant (cystadenocarcinoma) of BMC have previously been reported,4 and very few giant tumors have been described.3,4,10,16,19,20
They are slow-growing tumors with symptoms generally appearing as the cyst enlarges; and are occasionally discovered incidently on US, CT or endoscopic retrograde cholangiopancreatography (ERCP).3,18,21,22 The most frequent symptoms include pain and a sense of abdominal fullness or distension. Nausea and vomiting may develop as the result of cystic compression of the stomach and duodenum,1,8,19 and rarely an obstructive jaundice may occur as a result of compression of the biliary tree.3 Our patient developed ascites which can occur with cystic compression of the inferior vena cava or the hepatic veins.23 The tumors can also become inflamed, secondarily infected, and rupture. Other sequelae can include spontaneous bleeds into the cyst, the formation of calcifications and the creation of a fistula between the cyst and the biliary system.4,20,24 Malignant transformation has also been well documented with both subtypes;24.–27 patient age probably being a risk factor.7
BMC appears as a cystic, fluid-filled, well-circumscribed tumor, usually with multiple septae and/or internal papillary projections on US.20,28,29 CT generally shows a thin-walled cystic mass with contrast-enhancing internal septation, papillary infoldings and mural nodules.3,5 MRI sheds light on the nature of the intracystic fluid, and defines the cyst’s relationship with local vascular structures.30 ERCP and percutaneous transhepatic cholangiography (PTC) may demonstrate a fistula with the bile duct31 or filling defects, either due to compression by the tumor or the presence of mucin within the bile ducts.31,32 Very rarely mucin may even be seen extruding through the ampulla of Vater.33 Fine needle biopsy (FNB) may help to exclude an abscess or cystic metastasis within the liver,3 though the presence of haemorrhagic fluid is suggestive of malignancy.3 The presence of solid nodular masses, coarse calcification in the pseudocaspule and septae,4 and thickening of the cyst walls20 are also indicative of malignancy. CA 19-9 and other tumor markers may occasionally be elevated in the benign forms, and so are not especially useful in distinguishing between cystadenomas and cystadenocarcinomas. The only definitive way of determining any malignant potential is by examining the entire lesion for low-grade and/or high-grade/invasive components.
Histologically, BMC are divided into two subgroups. The first subgroup appears exclusively in women and comprises tumors with an ovarian stroma composed of 3 layers; the inner layer is lined with a single layer of columnar or cuboidal epithelium and an abundance of mucin producing cells, the intermediate layer comprises of a cellular subepithelial stroma of mesenchymal or spindle cells (known as the mesenchymal or ovarian stroma), and an outer layer consisting of connective tissue with hyalin which creates a pseudocapsule and separates the tumor from the adjacent liver parenchyma.8 The second subgroup has a similar composition but lacks an ovarian stroma and can occur in both males and females.
The differential diagnosis includes haematoma, a congenital solitary cyst, polycystic liver disease, cystic hamartoma, a post-traumatic cyst, liver abscess, necrotic neoplasm, hydatid cyst, Caroli’s disease and cystic metastases.3,34
Treatment regimes for BMC have included simple aspiration, sclerotherapy, laparoscopic fenestration,16 marsupialisation, internal drainage with a roux en-Y, and cyst excision.4 Recurrence rates are high, with 80% of cysts recurring after partial excisions and 100% following aspirations.35,36 Radical excision is the treatment of choice3,37 however even this modality is associated with recurrence rates of 10–20%.35,36,38 Radical excision is only feasible if the tumor is localised to one lobe or part of the liver, as the excision needs to include a 2 cm margin of normal liver tissue.20 This kind of extensive surgery is not possible in giant bilobar tumors (as was the case in our patient) and liver transplantation may be indicated.31,39,40 However, one must also balance the risk of malignancy, which has been estimated as around 20%,19 with the risk of liver transplantation and life-long immunosupressive therapy.
We felt that a subtotal cyst excision with closely monitored long-term follow-up was a reasonable alternative to transplantation, which we decided to hold in reserve should any signs of malignancy appear. Regardless of the treatment modality all BMC patients must be followed up over the longer-term, and our patient will have 6 monthly US, CT and CA 19-9; this surveillance becoming yearly after 3 years,19