Microbial translocation contributes to cirrhosis progression and complications. This study aims to investigate whether molecules related to intestinal permeability or microbial translocation can serve as prognostic biomarkers in patients with decompensated cirrhosis.
Materials and MethodsWe prospectively evaluated hospitalized patients with decompensated cirrhosis for liver function, complications during hospitalization, in-hospital mortality, composite outcomes of in-hospital mortality and complications, 12-month mortality, and survival rates. Blood samples were collected upon admission, and 1,3 beta-d-glucan, zonulin, calprotectin, and lipopolysaccharide-binding protein were measured using commercial kits.
ResultsNinety-one patients with decompensated cirrhosis were enrolled. The mean age was 58 ± 12 years; 57% were male. The three main cirrhosis etiologies were hepatitis C (35%), alcohol (25%), and non-alcoholic steatohepatitis (17%). In terms of liver function, 52% were Child C, and 68% had model for end-stage liver disease ≥15. The in-hospital and one-year mortality rates were 31% and 57%, respectively. Child-Pugh, 1,3 beta-glucan, and model for end-stage liver disease were positively correlated; zonulin was associated with complications during hospitalization (acute kidney injury) and composite outcomes, and calprotectin was associated with all outcomes except 12-month mortality.
ConclusionsSerum calprotectin and zonulin levels emerge as noninvasive prognostic biomarkers for potentially unfavorable outcomes in patients with decompensated cirrhosis.
Cirrhosis signifies the end stage of chronic liver diseases, including non-alcoholic fatty liver disease, alcohol-related liver disease, hepatitis B or C infection, and autoimmune diseases [1]. Cirrhosis usually develops following prolonged inflammation and tissue damage. This process causes the replacement of hepatic parenchyma with regenerative nodules and fibrosis, resulting in impaired liver function [2]. Overall, cirrhosis is a serious and progressive condition where patients are susceptible to various complications. The disease was responsible for more than 1.32 million deaths worldwide in 2017, corresponding to a global mortality rate of 2.4% [3]. Cirrhosis progresses from an asymptomatic compensatory stage to a symptomatic decompensated phase, marked by progressive liver failure, systemic inflammation, and portal hypertension. Following decompensation, both mortality and morbidity rise. Depending on the cause, the one-year mortality rate may reach 80% [4,5].
Recently, the intestinal microbiota has emerged as a crucial factor in the pathogenesis of extraintestinal diseases, particularly liver disorders. The liver consistently encounters a substantial load of microbial components and metabolites. Various conditions, including alcoholic and nonalcoholic liver disease, are linked to gut dysbiosis [6-8]. Common complications of advanced liver disease, such as hepatic encephalopathy (HE), show responsiveness to antimicrobial-based therapies, reinforcing the significant role of gut microbiota in liver diseases [9].
The gut-liver axis denotes a two-way communication link between the gastrointestinal (GI) tract and the liver, involving an intricate interplay of factors such as the intestinal barrier, portal and splanchnic circulation, cellular and vascular components of the hepatic sinusoids, and the bile duct system [10,11]. In patients with cirrhosis, there is commonly a disturbance in the gut-liver axis equilibrium, characterized by dysbiosis, heightened intestinal permeability, small intestinal bacterial overgrowth, diminished bile acid levels, hypochlorhydria, decreased gastrointestinal tract motility, and compromised immunological function of the intestinal and hepatic barriers [12,13]. As liver disease progresses, an escalation in intestinal permeability facilitates the passage of pathogens and their byproducts to the mesenteric lymph nodes and other extraintestinal organs. Elimination becomes challenging owing to immune changes associated with cirrhosis [14]. The abnormal translocation of microbes contributes to spontaneous infections observed in this population and fosters a persistent systemic inflammatory response that worsens the mechanisms underlying portal hypertension and cirrhotic complications [12,15].
Mounting evidence suggests that disruptions in the gut-liver axis, such as dysbiosis and heightened intestinal permeability, trigger oxidative stress and hepatic inflammation through Toll-like receptor signaling in hepatocytes. This process may contribute to the progression of chronic liver diseases[16]. Consequently, disturbances in the gut-liver axis play a pivotal role in liver disease development and advancement. Moreover, detecting microbial translocation could potentially serve as a diagnostic tool for early interventions. Therefore, this study aimed to investigate potential biomarkers of intestinal permeability and microbial translocation in cirrhosis. The primary objective of this prospective study was to assess 1,3-beta-d-glucan (BG), lipopolysaccharide-binding protein (LBP), calprotectin, and zonulin as potential biomarkers of intestinal permeability in cirrhosis. We also aimed to investigate their potential correlation with in-hospital mortality, cirrhosis complications, and overall survival.
2Materials and Methods2.1Study populationThis prospective observational study took place in three tertiary hospitals in Rio de Janeiro, Brazil, from April 2015 to December 2019. The study included adults with decompensated cirrhosis, irrespective of etiology, within 48 h of hospital admission. Decompensated cirrhosis was defined by stratifying the Child–Pugh score into two groups (B or C). Diagnosis of cirrhosis relied on clinical, radiological, and/or laboratory features. Exclusions encompassed patients under 18 years of age, those with hepatocellular carcinoma beyond the Milan criteria, individuals with a history of liver transplantation, those with compensated cirrhosis, or those with intestinal disease. All participants were followed up for one year.
2.2Study designAfter patient enrollment, peripheral blood samples were obtained within the first 48 h of hospital admission using ethylenediaminetetraacetic acid (EDTA) vacutainer tubes. The proposed biomarkers were detected, and routine blood tests, including complete blood count, coagulation panel, comprehensive metabolic panel, and ultrasensitive C-reactive protein, were conducted as general indicators of inflammation and/or infection. For the exploration of potential new biomarkers, samples underwent centrifugation at 1500 rpm for 10 min. Subsequently, 1-mL aliquots of serum were separated into cryotubes and stored at −80 °C for up to four months before readings. A photometric detection commercial kit (Fungitell® assay; Associates of Cape Cod, Inc., East Falmouth, MA, United States) was used to determine BG serum concentrations. This highly sensitive, microplate-based test detects serum BG in the 31–500 pg/mL range at 405 nm. A commercial enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotech Inc., Wayne, PA, United States) was used to measure LBP levels, and absorbance was measured at 450 nm. Serum zonulin concentrations were measured using a commercial human zonulin ELISA kit, with absorbance measured at 450 nm. Finally, calprotectin levels were measured using an ELISA kit (Biomatik, Wilmington, DE, United States). Upon hospital admission, clinical data and blood samples were collected to calculate Child-Pugh and model for end-stage liver disease (MELD) scores. The West Haven scale was used to diagnose and stage the severity of HE [17]. Throughout hospitalization, vigilant monitoring of complications, such as cirrhosis, infections, and the need for advanced life support, was conducted. All patients underwent follow-up for intrahospital clinical outcomes and one-year post-hospital discharge.
2.3Statistical analysisStatistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA). Numerical variables are presented as means, standard deviations, and medians, whereas categorical variables are expressed as absolute (n) and relative (%) frequencies. The Student's t-test for two independent samples was applied to compare differences in continuous variables concerning the distinct clinical outcomes. Spearman's rank correlation was used to assess relationships between continuous variables. Receiver operating characteristic (ROC) curves were constructed for biomarkers statistically associated with death and the composite outcome of complications or death. The objective was to identify optimal cutoff points to discriminate outcomes. Survival analysis was performed using the Kaplan–Meier curve and log-rank test for the death outcome. The follow-up time ranged from patient hospitalization to 12 months, utilizing the cutoff points identified by the biomarker ROC curves. All tests were two-tailed, and statistical significance was set at a P < 0.05.
2.4Ethical statementsThe Ethical Committee of the University Hospital of the Federal University of Rio de Janeiro, in collaboration with Quinta D'Or Hospital and Bonsucesso Federal Hospital, approved the study protocol (CAAE: 36,399,514.0.3002.5253). The study was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. Prior to inclusion in the study, all patients provided written informed consent.
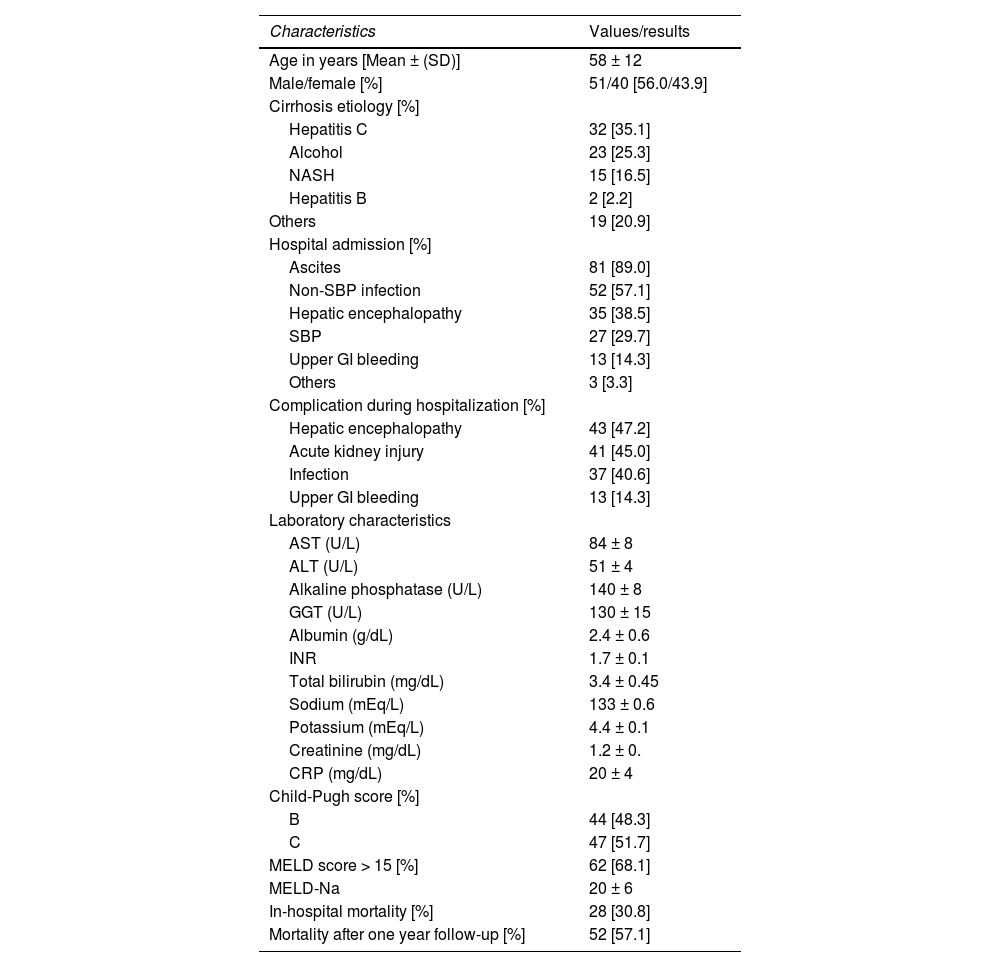
3Results3.1Patient baseline characteristicsWe enrolled 91 consecutive patients with decompensated cirrhosis. The clinical, demographic, and laboratory characteristics of the patients are detailed in Table 1. The mean age of the patients was 58 ± 12 years, with 57% being male. The three most common cirrhosis etiologies were hepatitis C (35%), alcoholic etiology (25%), and non–alcoholic steatohepatitis (17%). In terms of liver function, 52% were classified as Child C, whereas 68% had a MELD score ≥15, with a mean score of 20 ± 6. During hospitalization, 47% of the patients experienced HE, 45% had acute kidney injury (AKI), 40% had infections, and 14% had upper gastrointestinal bleeding. The in-hospital mortality rate was 31%, and the overall mortality rate after one year of follow-up was 57%.
Demographic, clinical, and laboratory characteristics of patients with cirrhosis included in the study (n = 91).
SD, standard deviation; NASH, nonalcoholic steatohepatitis; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; INR, international normalized ratio; CRP, C-reactive protein; MELD, model for end-stage liver disease; SBP, spontaneous bacterial peritonitis; GI, gastrointestinal tract.
When examining serum biomarker concentrations in relation to liver function, we identified a positive correlation between both BG (P = 0.002) and calprotectin (P < 0.001) concentrations and the MELD score. Similarly, BG (P = 0.001) and calprotectin (P = 0.01) exhibited correlations with liver function when stratified according to the Child-Pugh score. Elevated serum BG and calprotectin levels were noted in patients with deteriorating liver function and high MELD scores upon admission (Table 2).
Relationship between liver function and serum biomarkers.
BG, 1,3-beta-d-glucan; LBP, lipopolysaccharide-binding protein; CRP, C-reactive protein; MELD, model for end-stage liver disease. Data were analyzed using Spearman's rank correlation coefficient (CC).
During hospitalization, several complications were observed, including upper GI bleeding, HE, AKI, spontaneous bacterial peritonitis (SBP), and non-SBP infections. AKI and non-SBP infections were associated with elevated serum zonulin and calprotectin levels. Specifically, patients with AKI exhibited significantly higher levels of zonulin (41.7 vs. 23.6 ng/mL; P = 0.017) and calprotectin (1.4 vs. 1.2 ng/mL; P = 0.001) than those without AKI. Moreover, individuals with non-SBP infection displayed notably high serum calprotectin levels (1.39 vs. 1.09 ng/mL; P = 0.01) (Table 3). Notably, no discernible associations between the studied biomarkers and other investigated complications were observed.
Differences in serum biomarker levels concerning acute kidney injury and non-spontaneous bacterial peritonitis infections.
AKI, acute kidney injury; SBP, spontaneous bacterial peritonitis; BG, 1,3-beta-d-glucan; LBP, lipopolysaccharide-binding protein; SD, standard deviation. Data were analyzed using Student's t-tests.
The in-hospital mortality rate was 31% and highest in patients with elevated serum calprotectin concentrations upon admission (1.5 vs. 1.1 ng/mL; P = 0.004). However, no additional associations were identified between in-hospital mortality and the other biomarkers, as illustrated in Table 4.
Differences in serum biomarker levels concerning in-hospital mortality and the composite endpoint of complications or in-hospital mortality.
BG, 1,3-beta-d-glucan; LBP, lipopolysaccharide-binding protein; SD, standard deviation. Data were analyzed using a Student's t-test.
Upon examining the combined occurrence of complications and in-hospital mortality in patients with cirrhosis, we observed elevated zonulin (35 vs. 16 ng/mL, P = 0.001) and calprotectin (1.3 vs. 0.9 ng/mL, P = 0.023) levels in those experiencing either outcome (Table 4).
3.6Association of serum biomarker concentrations with the 12-month mortalityNo association was observed between 12-month mortality and serum biomarker levels (Supplementary Table S1).
3.7Association of serum biomarker concentrations with survivalAmong the investigated serum biomarkers, calprotectin demonstrated the strongest correlation with survival in patients with decompensated cirrhosis. Kaplan–Meier survival curves were generated for 12-month survival, utilizing the optimal cutoff point for serum calprotectin set at 1.35 ng/mL (area under the curve: 0.688; 95% confidence interval: 0.545–0.796; P = 0.012) (Table 5). Patients with serum calprotectin levels below 1.35 ng/mL exhibited significantly longer survival than patients with levels equal to or greater than 1.35 ng/mL (P = 0.007) (Fig. 1). None of the other biomarkers analyzed in this study showed a significant association with survival.
Assessment of mortality in relation to serum biomarkersa.
In this study, we conducted a prospective observational investigation to assess the potential of non-invasive serum tests as biomarkers for predicting liver dysfunction, disease-associated complications, in-hospital mortality, and one-year survival in patients with decompensated cirrhosis. Overall, the performance of serum zonulin and calprotectin in the context of decompensated cirrhosis was favorable, thereby highlighting the crucial role of the gut-liver axis in cirrhosis progression via molecules associated with intestinal permeability and systemic inflammation.
In the context of cirrhosis, gut microbial translocation is associated with most complications in affected patients [18,19]. Therefore, understanding the pathophysiology of microbial translocation in cirrhosis is crucial for successful early interventions and improvements in clinical outcomes [20]. In this regard, we employed accessible commercial laboratory tests that are reproducible with simple application. The goal was to identify new non-invasive biomarkers capable of early detection of complications and clinical outcomes, potentially enhancing the prognosis of patients with cirrhosis. Among the tests directly related to gut microbiota, we explored serum LBP concentrations. Associations between LBP, bacterial translocation, severe bacterial infection [21,22], hemodynamic instability [23], prediction of infection, and mortality in patients with cirrhosis [24] have been demonstrated. However, in the current study, LBP did not show significant associations with the outcomes analyzed.
In patients diagnosed with inflammatory bowel disease, elevated serum BG concentrations were detected in correlation with disease activity [25]. Moreover, alterations in the intestinal barrier cause increased translocation of bacterial and fungal components, thereby heightening infection risk [26,27]. Therefore, the current study's focus was determining BG levels in patients with decompensated cirrhosis. BG constitutes a ubiquitous cell wall component of microorganisms within the gut microbiota, including Candida and Aspergillus spp. [28]. The molecule has been linked to disease outcomes in sepsis [29] and patients infected with HIV [30]. However, contrary to a recent study by Egger et al., where patients with cirrhosis exhibited elevated BG levels associated with mortality and inflammation markers [31], the current study did not reveal any significant association between serum BG levels and disease outcomes. Distinct results may be attributed to variations in sample characteristics, such as the number of patients, their origins, and etiological diagnoses. In contrast, the current study suggests an association between BG, Child-Pugh, and MELD classifications. This suggests a potential link between fungal dysbiosis, chronic liver disease development [32,33], and liver dysfunction progression [34].
Zonulin is a protein precursor of haptoglobin-2 synthesized by intestinal and liver cells [35] and is secreted by epithelial cells upon exposure to enteric bacteria. The precursor attaches to special intestinal epithelial cell membrane receptors, disrupting tight junctions and increasing gut permeability [36]. An association between elevated serum zonulin concentrations and abnormalities in intestinal permeability have been observed in conditions such as diabetes [37], obesity [38], and inflammatory bowel disease (IBD) [39]. To investigate the potential impact of zonulin levels on patient prognosis, zonulin levels were measured in the study cohort of patients with cirrhosis. Consistent with a previous study, we identified zonulin as a reliable indicator of outcomes in patients with decompensated cirrhosis [40]. Specifically, we observed a positive association between serum zonulin levels and the development of complications or death during hospitalization. Notably, a significant association between high zonulin levels and AKI is highlighted among the complications. This finding supports the role of increased intestinal permeability and microbial translocation as underlying mechanisms capable of inducing kidney dysfunction in cirrhosis.
Finally, we explored calprotectin, a neutrophil-derived protein highly resistant to degradation by intestinal and bacterial enzymes. The protein is routinely measured in fecal samples [41] and is associated with intestinal inflammation and disease activity in IBD [42,43]. The rationale for incorporating calprotectin in this study was the potential for its serum levels to reflect intestinal or systemic inflammation concurrently and increased intestinal permeability, which are relevant to the analyzed outcomes. Although only a few studies have delved into calprotectin as a biomarker for liver cirrhosis, a positive correlation between fecal calprotectin and hepatic encephalopathy has been identified [44]. Additionally, ascitic fluid calprotectin has been linked to the development of SBP [45-47]. Recently, elevated serum calprotectin levels have been associated with the prognosis of patients experiencing acute decompensation without acute-on-chronic liver failure [48]. In this study, we attempted to explore the relationship between serum calprotectin, complications of cirrhosis, in-hospital mortality, and one-year survival. Among the biomarkers analyzed, serum calprotectin exhibited the strongest associations with all studied outcomes, except for 12-month mortality, which was evaluated during the follow-up of patients.
Nevertheless, our study has certain limitations, including the limited number of patients and its cross-sectional nature. An increased sample size could have facilitated the cutoff value assessment involving additional markers, apart from calprotectin, for predicting complications and mortality in cirrhotic patients. Additionally, incorporating follow-up data and serial measurements could have provided a precise evaluation of whether changes in serum biomarker levels could indicate early complications and death, either intra-hospital or during the one-year follow-up period. Despite these limitations, it is essential to note the scarcity of studies that analyze both serum calprotectin and serum zonulin among patients with decompensated cirrhosis. We acknowledge that further research is necessary to validate the findings of the current study. Nevertheless, the results strengthen the notion that gut-related pathophysiological phenomena underlie the decompensation of chronic liver diseases, likely attributed to increased intestinal permeability, dysbiosis, and microbial translocation. Importantly, our study may positively contribute to the management of patients with decompensated cirrhosis by reducing complications and mortality. Although additional studies are required to confirm our findings, the results suggest a potential link between gut-related factors and chronic liver disease progression.
5ConclusionsAmong the investigated biomarkers, BG was positively associated with the severity of liver disease. High levels were observed in patients with elevated MELD scores and advanced liver disease, as classified by the Child-Pugh classification. Zonulin demonstrated a positive association with AKI, a major complication of liver cirrhosis, as well as the composite endpoint of complications and in-hospital mortality. Calprotectin showed a significant positive correlation with all outcomes in our study except for 12-month mortality.
FundingCoordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001, National Council for Scientific and Technological Development (CNPq) (306,634/2019–8), and the FAPERJ (Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro) (E26/202.781/2017, E-26/010.001279/2015, E-26/203.061/2016).
Author contributionsCMAB, KFS, RMP, and HSPS participated in the conceptualization of the study; CMAB, EAV, JPLM, KFS, SLBR, PTS, AMP, GP and FFF participated in the acquisition, analysis, and interpretation of data; CMAB and EAV participated in the drafting of the manuscript; RMP and HSPS obtained funding, analyzed, and interpreted the data, and critically revised the manuscript for important intellectual content. All authors provided final approval for the submitted version of the manuscript.
Data availability statementThe technical appendix, statistical code, and dataset supporting the conclusions of this study will be made available by the corresponding author [hsouza@hucff.ufrj.br] without undue reservation upon request.
The authors thank the Brazilian research foundations, CAPES, CNPq, and FAPERJ, for their financial support. We would like to thank Editage (www.editage.com.br) for English language editing.