Hepatitis C virus (HCV) infection is associated with the persistence of viremia in most infected individuals in the presence or absence of progressive liver disease. The coinfection with human immunodeficiency virus (HCV/HIV) has been associated with accelerated progression to severe liver injury compared to HCV monoinfected patients [1]. In the United States and Europe, approximately 33% of all HIV-infected persons are coinfected with HCV. This percentage may vary depending on the geographical region and mode of acquisition of HIV, being higher among intravenous drug users [2]. Although the underlying mechanisms remain unclear, HIV infection alters the natural history of HCV disease. It is associated with an increased likelihood of chronicity following acute infection, accelerated progression to end-stage liver disease, and increased risk of liver-related deaths(1). Different pathogenetic factors contribute to liver disease progression in HIV/HCV coinfection, including quantitative decline in CD4+ and CD8+ T-cells responses to HCV infection, qualitative changes in immune responses as evaluated by cytokine polarization profile and impaired dendritic cell function. In addition, host and virus genetic factors may influence the evolution of progressive liver disease [3].
The classical class I HLA (HLA-A, HLA-B, and HLA-C) molecules play an essential role in presenting viral peptides to cytotoxic CD8+ T-cells [4]. Some viruses, including cytomegalovirus, have developed the ability to decrease the expression of these molecules as a powerful evasion mechanism of the lytic activity of T CD8 cells [5]. Virus-infected cells lacking HLA classical class I molecules are usually attacked by cytotoxic NK cells, which detect this membrane abnormality. As a strategy to evade the action of cytotoxic NK cells, some viruses induce the expression of non-classical HLA (HLA-E, HLA-F, and HLA-G) molecules, whose central role is to regulate the function of immune system cells [6]. In this context, HLA-G has a well-recognized property to down-regulate the immune response, binding to inhibitory receptors (ILT-2, ILT-4, KIR2DL4, and others) present on antigen-presenting cells, CD4+ and CD8+ T lymphocytes, and NK cells. Inhibition of the immune response may be beneficial, as in autoimmune disorders and transplantation, but may produce detrimental effects in malignancies and chronic viral diseases, in which a solid cytotoxic immune response is required to eliminate malignant and chronically virus-infected cells [7].
HLA-G was first detected in cytotrophoblast cells at the maternal-fetal interface, where they maintain a tolerogenic status between the mother and the semiallogenic fetus. In pathological conditions, HLA-G has been detected in several types of tumor, allografts, inflammatory and autoimmune disorders, and virus diseases, including those caused by influenza, rabies, cytomegalovirus, herpes, and HIV [8]. Increased soluble HLA-G level is detected in the very early stage of HIV infection, and its production has been associated with an increased rate of disease progression [9]. Concerning hepatotropic viruses, our group reported a high percentage of HLA-G expression in hepatocyte and biliary epithelial cells of biopsy samples obtained from hepatitis B [10] and HCV-infected patients [11]. Considering that the coinfection of HIV/HCV has been associated with worsening liver damage and that HLA-G down-regulates the immune response against viruses, we hypothesized that HLA-G expression could be associated with impaired liver and patient morbidity. Then, we analyzed HLA-G expression in liver specimens of HIV/HCV-coinfected subjects and stratified the findings according to clinical and histopathological features.
2Patients and Methods2.1SamplingIt included 59 chronically HCV/HIV-coinfected patients followed at the School of Medicine of Ribeirão Preto, University of São Paulo (FMRP-USP), São Paulo, Brazil, from 2002 to 2006. Liver biopsies and blood samples were obtained during the follow up. Epidemiologic data clinical and laboratory results were retrieved from the patients' medical records. Fifty patients had been submitted to hepatitis C antiviral therapy with pegylated-IFN-α (PEG-IFN-α) plus Ribavirin, according to recommendations for treatment at that time.
2.2Histological analysesLiver tissue samples were obtained by using the percutaneous biopsy of the right hepatic lobe with a 14-gauge Tru-cut needle guided by ultrasound. After performing the biopsy, patients remained in the hospital to monitor vital signs for 6 hours.
Histologic analyses of liver samples were performed by using 5-µm-thick paraffin-embedded liver sections stained with hematoxylin/eosin, Masson-trichrome, and Prussian blue. The magnitude of fibrosis degree and the histological activity index (HAI) were assessed according to the criteria described by Knodell et al. modified by Desmet et al. [12], which classify liver involvement according to the necroinflammmatory activity (graded 0-18) and severity of fibrosis (stage F0–F4). Iron deposit semi-quantitative analysis was performed using Perl's staining and classified according to Sciot et al. [13]. We graded hepatic steatosis according to the method of Patton et al.[14]. All biopsies were assessed and classified in a blind protocol by an experienced pathologist specialized in liver diseases.
2.3HIV viral load and HCV RNA detectionHIV viral load was determined directly by a hybridization sandwich nucleic acid technique using the VERSANT1 HIV-1 RNA 3.0 (bDNA) (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA) protocol, according to the manufacturer's instructions. Values less than 50 copies/ml were reported as undetectable. A negative control was included in each run.
HCV-RNA detection was determined by the polymerase chain reaction (PCR) (commercial COBAS AMPLICOR HCV method, v2.0, Roche Molecular Systems, Branchburg, USA); sensitivity limit of 50 IU/mL). Response to HCV therapy, also known as sustained virologic response (SRV), was defined as undetectable HCV-RNA in plasma 6 months after the end of treatment with PEG-IFN-α and Ribavirin.
2.4Immunohistochemistry assay for HLA-GAdditional 5-µm-thick sections were placed on organosilane-pretreated slides for HLA-G immunohistochemical assay (IHA) using two HLA-G monoclonal antibodies: 5A6G7 (EXBIO, Vestec, Czech Republic) against soluble HLA-G5 and HLA-G6 isoforms, and 4H84 (kindly provided by Mc-Master, San Francisco, CA), which recognize all soluble and membrane-bound HLA-G molecules. The IHA was performed as reported elsewhere [15], using an antibody dilution of 1:50. As a positive control, paraffin-embedded sections of trophoblast tissue obtained from spontaneous abortions at approximately 12 gestational weeks were systematically used. A negative control was performed by omitting the primary antibody. The experienced pathologist assessed the magnitude of HLA-G expression in a qualitative way.
2.5Statistical analysesStatistical analyses were performed using the Student t or nonparametric tests to compare continuous variables. Pearson's χ2 test corrected by the Yates method and Fisher's exact test were used, when appropriate, to compare proportions between HLA-G-positive and -negative groups. p values <0.05 were considered significant.
2.6Ethical statementThe study protocol was approved by the local Ethics Research Committee (protocol number 4916/2010) and all the participants gave written informed consent to participate. As an exclusion criterion, patients needed to be free of hepatitis B infection.
3ResultsDemographic, laboratory, and histologic findings of patients are shown in Table 1. Genotype 1 was the most prevalent (88%), especially subgenotype 1a (61%), followed by genotype 3a (10%) and genotype 2a (2%).
Demographic, HCV genotype and liver histological characteristics of 59 HIV-HCV coinfected subjects
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 45 | 76.3 |
| Female | 14 | 23.7 |
| Population group | ||
| Caucasian | 49 | 83.1 |
| Non-Caucasian | 10 | 16.9 |
| Age (years) | ||
| Mean (±SD) | 37.7 (±5.53) | - |
| Median (range) | 38 (25-50) | - |
| HCV Genotype | ||
| 1 (not subtyped) | 2 | 3.3 |
| 1a | 36 | 61.1 |
| 1b | 9 | 15.2 |
| 1a/1b | 5 | 8.5 |
| 2 | 1 | 1.7 |
| 3a | 6 | 10.2 |
| Liver fibrosis | ||
| F1 | 24 | 40.7 |
| F2 | 17 | 28.8 |
| F3 | 13 | 22.0 |
| F4 | 5 | 8.5 |
| Liver inflammatory activity | ||
| 0 – 9 | 26 | 44.1 |
| > 9 | 33 | 55.9 |
| Liver iron deposition | ||
| No | 29 | 49.2 |
| Yes | 30 | 50.8 |
| Liver Steatosis | ||
| No | 43 | 72.8 |
| Yes | 16 | 27.2 |
According to the severity of hepatic fibrosis, 24 cases (41%) were classified as F1, 17 cases (29%) as F2, 13 cases (22%) as F3 and 5 cases (8%) as F4. For statistical analyses, the degree of fibrosis was compared between groups and between mild (F1 and F2) and severe (F3 and F4) forms. Median ages of the patients at liver biopsy were 36.5 years for F1, 37 years for F3 and 38 years for F2 and F4. According to the necroinflammatory activity, 26 (41.1%) patients exhibited no or mild activity (0 to 9), and 33 patients (55.9%) showed moderate or severe (>9) activity.
The profile of HLA-G staining was similar for both monoclonal antibodies used in this study. HLA-G was strongly expressed in all trophoblast specimens used as positive controls. In the HCV/HIV coinfected patients, HLA-G molecules were detected in 38 cases (64%), and expression was primarily observed in hepatocytes and in biliary epithelial cells. HLA-G expression exhibited both focal and diffuse liver distribution and occurred irrespective of zones 1, 2, and 3 of the hepatic acinus. Figure 1 illustrates different patterns of HLA-G expression in liver biopsy samples.
HLA-G expression in liver biopsy of HIV/HCV coinfected patient analyzed by immunohistochemistry. (A) sample with no expression of HLA-G. (B) sample with mild HLA-G expression. Thicker circle shows an area with hepatocytes labeled by anti-HLA-G antibody (thick arrow). Thinner circle shows an area with non-labeled hepatocytes (thin arrow). (C) marked and diffuse expression of HLA-G in hepatocytes.
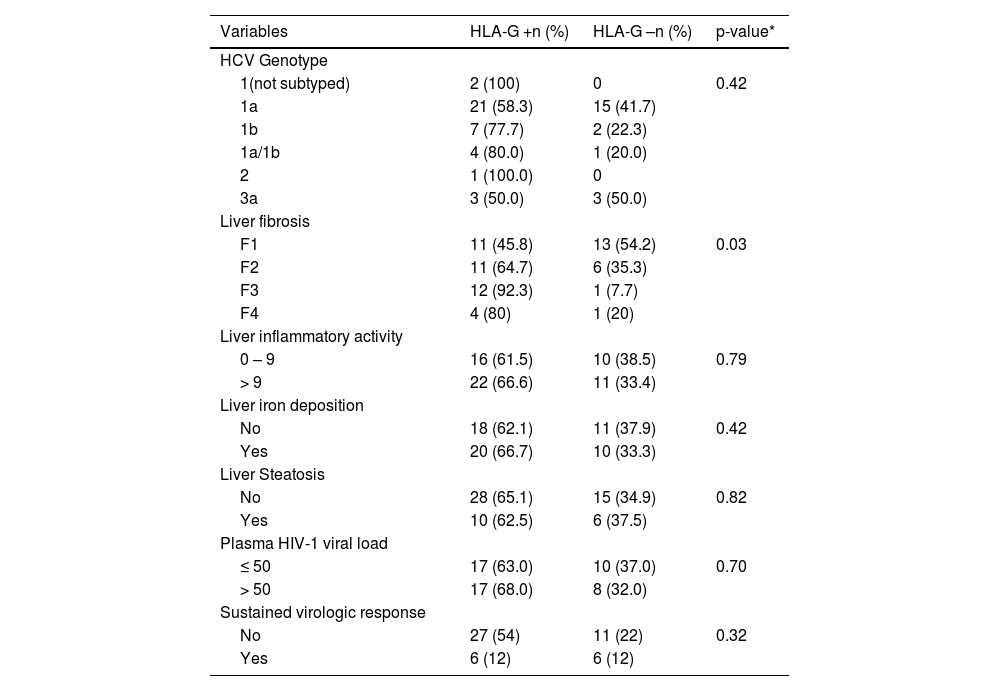
Table 2 shows the analyses of HLA-G expression in HIV/HCV liver specimens, stratified according to laboratory and histological features, as well as response to PEG-IFN-α plus Ribavirin therapy. Considering the severity of liver fibrosis and comparing the expression or not of HLA-G in each group of fibrosis, the HLA-G expression increased as long as increased the fibrosis degree (45.8% for F1; 64.7% for F2; 92.3% for F3; 80% for F4; p = 0.03). Comparing more severe fibrosis stages (F3 and F4) versus milder fibrosis (F1 and F2), HLA-G expression was more prominent in the first group (F3 and F4) (94,1% x 55%; p<0.01) (not shown in the table). No association was observed between the HLA-G distribution patterns and the severity of liver necroinflammatory activity or response to hepatitis C therapy.
Virological and histological features, according to liver HLA-G expression, in HIV-HCV coinfected subjects
| Variables | HLA-G +n (%) | HLA-G –n (%) | p-value* |
|---|---|---|---|
| HCV Genotype | |||
| 1(not subtyped) | 2 (100) | 0 | 0.42 |
| 1a | 21 (58.3) | 15 (41.7) | |
| 1b | 7 (77.7) | 2 (22.3) | |
| 1a/1b | 4 (80.0) | 1 (20.0) | |
| 2 | 1 (100.0) | 0 | |
| 3a | 3 (50.0) | 3 (50.0) | |
| Liver fibrosis | |||
| F1 | 11 (45.8) | 13 (54.2) | 0.03 |
| F2 | 11 (64.7) | 6 (35.3) | |
| F3 | 12 (92.3) | 1 (7.7) | |
| F4 | 4 (80) | 1 (20) | |
| Liver inflammatory activity | |||
| 0 – 9 | 16 (61.5) | 10 (38.5) | 0.79 |
| > 9 | 22 (66.6) | 11 (33.4) | |
| Liver iron deposition | |||
| No | 18 (62.1) | 11 (37.9) | 0.42 |
| Yes | 20 (66.7) | 10 (33.3) | |
| Liver Steatosis | |||
| No | 28 (65.1) | 15 (34.9) | 0.82 |
| Yes | 10 (62.5) | 6 (37.5) | |
| Plasma HIV-1 viral load | |||
| ≤ 50 | 17 (63.0) | 10 (37.0) | 0.70 |
| > 50 | 17 (68.0) | 8 (32.0) | |
| Sustained virologic response | |||
| No | 27 (54) | 11 (22) | 0.32 |
| Yes | 6 (12) | 6 (12) |
* P-value of Yates corrected chi-square
Iron staining and detection of hepatic steatosis, performed in 100% specimens, were positive in 30 (51%) and 16 (27%), respectively. No association was detected between liver HLA-G expression and iron deposits or hepatic steatosis. All HCV/HIV coinfected patients exhibited detectable HCV viremia. HIV-1 viral load was available for 52 patients (88.1%) and was performed within the last six months before the hepatic biopsy. Twenty-seven patients (51.9%) had an undetectable viral load. No association was observed regarding HLA-G liver expression and age, sex, population group, HCV genotype and HIV viral load.
4DiscussionIn low- and middle-income countries, HCV infection represents a significant problem in persons infected with HIV. With the evolution of antiretroviral therapy for HIV over the years, there has been a progressive decline in mortality from causes related to AIDS, with a proportional increase in mortality from liver disease, mainly due to chronic viral hepatitis B and C [1].
The role of cell-mediated immune response and its relationship to sustained HCV control in the context of HIV infection remains unclear. Available information about the immunological variables associated with the outcome of HIV/HCV-coinfection is scarce. Previous studies revealed that CD4 T cell depletion induced by HIV infection is associated with loss of adaptive HCV-specific immune responses. In fact, in HCV mono-infection, most patients who spontaneously resolve HCV generate and maintain vigorous peripheral lymphoproliferative responses to HCV antigens [16,17].
A more rapid progression of liver disease in HIV/HCV coinfected patients is well documented in several studies. [1,16,18]. Different factors contribute to the accelerated evolution of severe liver disease. The central among these factors is HIV-induced immunosuppression, which may induce liver modifications of cytokine polarization patterns towards a Th2 response [19]. Also, viruses that infect humans have developed many mechanisms for evading the immune system. For instance, HIV Nef protein differentially down-regulates the expression of HLA-A and HLA-B, but not of HLA-C, HLA-E, or HLA-G molecules, on the surface of infected cells. [20] Moreover, HIV infection leads to increased HLA-E and HLA-G expression, reducing NK cell-mediated cytotoxicity activity [21]. HLA-G expression has been described in cells infected by HIV, cytomegalovirus, rabies, and herpes simplex [7,8].
The present study observed that HLA-G expression occurs primarily in the later stages of fibrosis in the HIV/HCV coinfection. This finding was similar to the other research of our group, which described that the HLA-G expression increased with the progression of liver fibrosis, both in HCV-monoinfected and HIV/HCV-coinfected patients [22]. Previously, Amiot et al. [23] had described a positive correlation between HLA-G+ cell numbers and fibrosis area in the liver among HCV monoinfected patients.
The presented results suggest that HLA-G expression may play a role in the mechanisms that facilitate disease evolution and may participate in the deterioration of the immune response against HCV in the coinfection. Several observations support this idea: i) increased plasma levels of soluble HLA-G and IL-10, an inducer of HLA-G expression, are reported in patients with chronic HCV infection [24], ii) high levels of soluble HLA-G and IL-10 are reported in HIV rapid progressors [9], iii) cytokines associated with the Th-2 cytokine profile may contribute to liver fibrosis in HIV patients [3], iv) the expression of HLA-G has been associated with the shift to a Th2 cytokine polarization [7].
In the present study, HLA-G expression in the liver was not correlated to antiviral response to hepatitis C therapy with PEG-IFN-α plus ribavirin. To our knowledge, this is the first work regarding HLA-G liver expression and response to PEG-IFN-α based therapy. Murdaca et al. [25] observed that basal serum levels of soluble isoforms of HLA-G antigens were similar among HCV positive responders (SVR) and non-responders (NR) to PEG-IFN-α and ribavirin. However, the levels of soluble HLA-G molecules decreased in both groups after three months of HCV therapy but remained higher in NR patients than in SVR patients.
No patient in the present study was treated with the new Direct-Acting Antiviral (DAA) agents, as these medications were unavailable when the patients were treated. DAAs have dramatically changed the panorama of chronic hepatitis C, with cure rates of more than 90% [26]. Initial studies have alerted for increasing rates of hepatocellular carcinoma (HCC) after treatment with DAAs [27–29]. It has been proposed that ceasing HCV infection may induce relevant modifications in the hepatic immune anti-tumor response [28]. However, other more extensive studies have not confirmed these results [30–35]. Amaddeo et al. [36] showed that in patients with compensated cirrhosis who underwent surgical resection for hepatocellular carcinoma and HCV infection cured by therapy with DAAs, intra‐hepatic inflammation and immune responses persist after treatment. To our knowledge, no study has described the correlation between HLA-G expression in the liver or serum and response to DAA-based therapy.
5ConclusionsIn conclusion, the present study is one of very few to explore the HLA-G expression profile in the liver microenvironment of HIV/HCV-coinfected patients, in whom more than half of the biopsies (64%) exhibited HLA-G expression. The presence of HLA-G was significantly associated with later stages of fibrosis. It was not influenced by patient age, sex, population group, HCV genotype, HIV viral load, iron deposits, and hepatic steatosis.
Author contributionsFernando Crivelenti Vilar: conceived the study, collected and analysed the data, and wrote the manuscript; Rodrigo de Carvalho Santana: collected and analysed the data, wrote the manuscript; Janaina Cristiana de Oliveira Crispim: data analysis, study design, and draft the manuscript; Ana Letícia Gomide Zanin Borducchi: collected and analysed the data; Eduardo Antonio Donadi: conceived the study, collected and analysed the data, wrote the manuscript; Benedito Antonio Lopes da Fonseca: conceived the study, analysed the data and wrote the manuscript.
All authors have contributed to the critical revision and writing of the paper. All authors read and approved the final manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.