Type I interferons are potent cytokines that possess antiviral, immunomodulating and antiproliferative actions. The development of autoimmune hepatitis is a well recognized complication of treatment with alpha IFN in patients with chronic viral hepatitis. Yet, the occurrence in patients under treatment with beta IFN for other indications is controversial and its occurrence often underestimated. We report two cases of severe acute autoimmune hepatitis in two patients undergoing therapy with IFN beta 1a for multiple sclerosis who recovered under early immunosuppressive therapy.
Type I interferons are potent cytokines that possess antiviral, immunomodulating and antiproliferative actions.1 Both recombinant, natural and pegylated alpha IFNs are approved for treatment of chronic hepatitis C and B, condyloma acuminatum, Kaposi’s sarcoma in HIV positive patients and other malignancies. IFN beta has been approved by the FDA for the treatment of relapsing and relapsing-remitting multiple sclerosis (RRMS) where it has proven to reduce disease activity, progression and relapse rate.2,3 Interferons have important adverse effects that may limit their use. Side effects include exacerbations of preexisting autoimmune diseases or induction of autoimmunity, attributed to the immunomodulatory properties of IFNs.4 The development of autoimmune hepatitis is a well recognized complication of treatment with alpha IFN in patients with chronic viral hepatitis.5 Yet, the occurrence in patients under treatment with beta IFN for other indications is controversial and its occurrence often underestimated.
We report two cases of severe acute autoimmune hepatitis in two patients undergoing therapy with IFN beta 1a for multiple sclerosis who recovered under early immunosuppressive therapy.
Case ReportCase 120-year-old woman with diagnosis of relapsing-remitting MS, who developed 18 months before admission facial palsy and dyplopia, initiating treatment with 5 intermittent boluses of methylprednisolone (1 g/each), followed by IFN Beta 1a and tapering of prednisone. Due to recurrent relapses, she received two additional cycles with steroids and Beta IFN twelve and six months before entry. One year before admission IFN had to be temporarily discontinued due to an elevation of transaminases x 3. Treatment was reinstituted once she recovered normal AST and ALT levels. The patient remained asymptomatic until 2 months before entry, when she developed right hemiparesia and homolateral hypoesthesia. She was treated with 3 additional boluses of methylprednisolone and started a new cycle of IFN beta 1a. Three weeks later she presented an elevation of transaminases x 5 and bilirrubin x 3. IFN was discontinued. During the following week she started with progressive jaundice, asthenia, abdominal discomfort and nausea. Liver function tests showed total bilirrubin 16.1 mg/dL, AST 670 UI/dL, ALT 710 UI/dL, alkaline phosphatase 192 UI/dL and gamma-glutamyl transpeptidase 64 UI/Dl (Table 1). The patient had no previous history of liver disease and she did not consume alcoholic beverages, illicit drugs or any other hepatotoxic medications, with the exception of occasional ibuprofen. Pre-treatment liver function tests were normal. Viral serologies for hepatitis A, B and C, citomegalovirus and Epstein Barr virus were negative.
Laboratory data of case 1.
| Case 1 | - 12 months | - 5 weeks | - 4 weeks | Admission | + 2 days | + 10 days |
|---|---|---|---|---|---|---|
| Hb (gr/dL)/ White cell count (mill/mm3) | 13.7/4,800 | 12.9/5,580 | 13.1/5,420 | 13.3/6,220 | 12.7/9,740 | 12.3/8,790 |
| Glycemia (mg/dl) | 79 | 82 | 78 | 72 | 89 | 91 |
| Creatinine (mg/dL) | 0.7 | 0.6 | 0.7 | 0.7 | 0.8 | 0.6 |
| Total/Dir Bil (mg/dL) | 0.9/0.7 | 3.9/2.8 | 16.1/12.7 | 19.3/15.7 | 17.1/12.2 | 7.2/5.9 |
| AST/ALT (< 40 UI/L) | 105/128 | 191/218 | 670/710 | 580/605 | 370/410 | 86/92 |
| Alk Phosph (< 100 UI/L) | 96 | 101 | 192 | 156 | 114 | 109 |
| GGT (< 49 UI/mL) | 32 | 42 | 64 | 59 | 53 | 56 |
| Quick time (%) | 96 | 82 | 77 | 19 | 21 | 67 |
| Factor V (%) | - | - | - | 27 | 43 | 76 |
| Gammaglob (g/dL) | - | - | 3.3 | 3.1 | 2.8 | 2.1 |
Anti smooth muscle antibody was positive in low titers (1/40), with negative antinuclear antibody and anti LKM 1 antibodies, as well as antimitochondrial antibody, anticardiolipins IgG and IgA, anti gastric parietal cell and ANCA. Copper metabolism was normal. An ultrasound with Doppler study of the hepatic vessels was unremarkable.
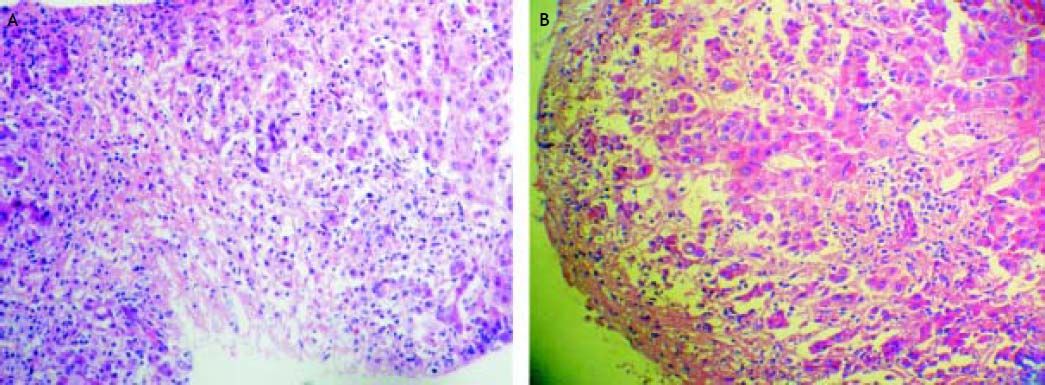
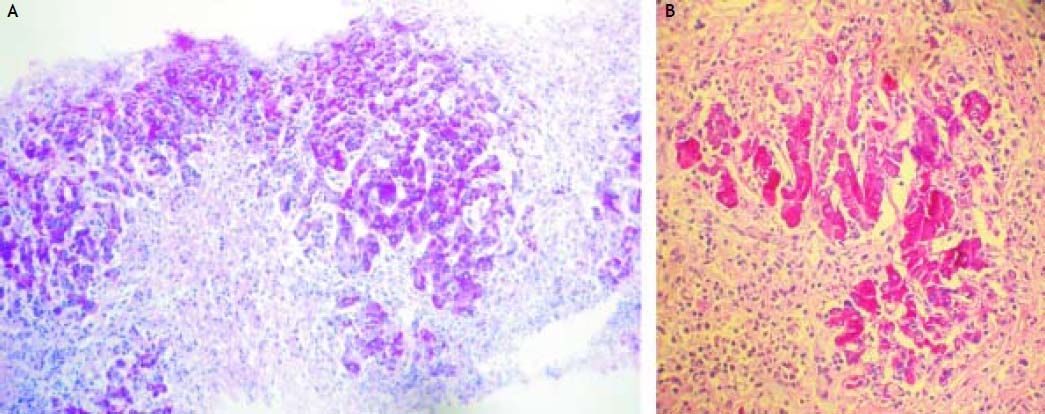
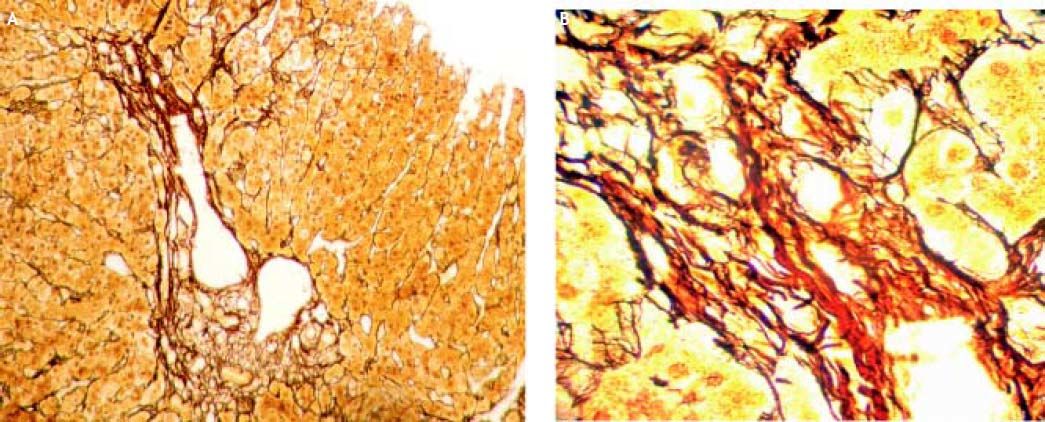
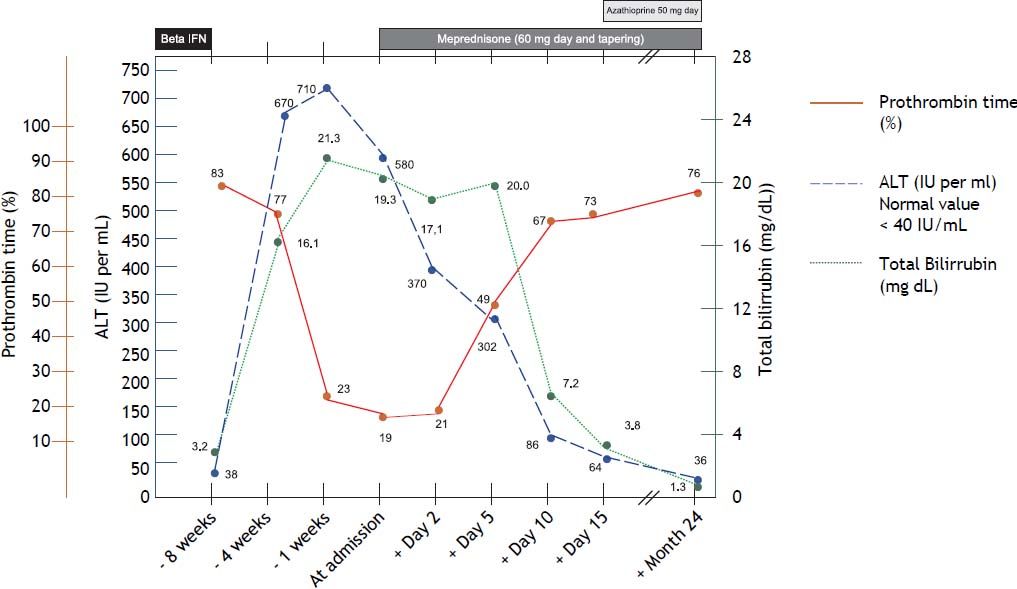
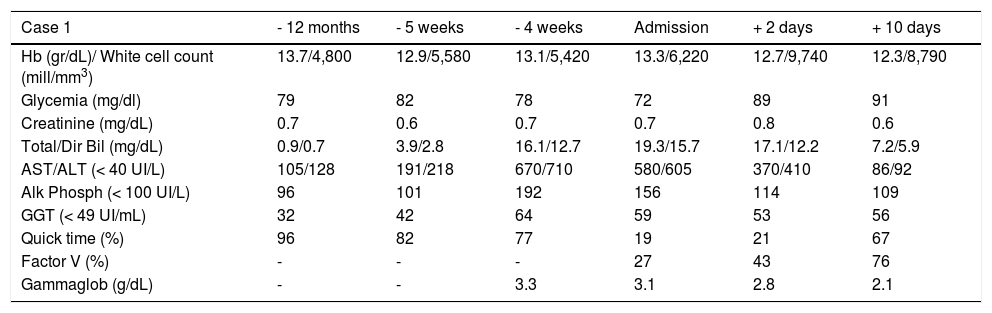
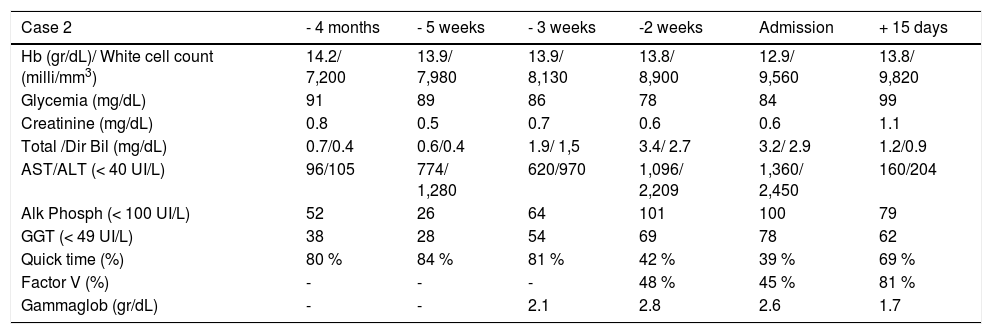
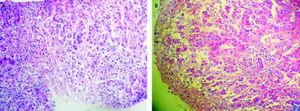
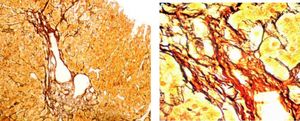
Three weeks after jaundice, she abruptly developed decreased level of consciousness, asterixis and a fall in prothrombin time to 23%, with a coagulation factor V of 27 %. She was transferred to our Liver Transplantation Unit within 6 h of encephalopathy and admitted to the Intensive Care Unit. A transjugular liver biopsy was performed 4 h post-admittance that showed submassive hepatocellular necrosis with marked portal and lobular inflammation and parenchymal collapse, interface hepatitis with abundant plasma cells and rosette formation (Figures 1-3). Gammaglobulin level was 3.3 mg/dL.
The patient quickly progressed to grade III encephalopathy and was listed for liver transplantation. Prednisone was started via a nasogastric tube at a dose of 1 mg/kg (60 mg/day). By day 2 post-corticosteroids her liver function started to improve, she had no evidences of encephalopathy and presented complete normalization of prothrombin time and factor V by day 10 post treatment (Figure 4). She was started on azathioprine (50 mg/day) on day 15 and steroid tapering and remained with normal liver function tests for the following 24 months. A new liver biopsy was performed showing no inflammatory activity and minimal portal fibrosis 2 years after the beginning of the disease. The patient was successfully weaned from immunosuppression 3 years after beginning with corticosteroids, with the exception of 2 additional cycles of pulses of corticosteroids due to two relapses of her MS, that presented as right hypoesthesia associated with facial palsy without evidence of relapse of autoimmune hepatitis. On follow up she developed positivity for ANA (1/320) (homogeneous pattern) and anti-smooth muscle (1/160), anti-actin +. Eight months after starting immunosuppressive treatment she developed hypothyroidism with associated antithyroglobulin and anti thyroperoxidase antibodies in high titers and is currently replaced with levothyroxin 100 μgr/day.
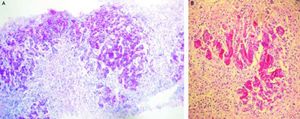
Case 247-year-old man with diagnosis of relapsing-remitting MS who started treatment with IFN beta 1a forty-one months before admittance. Four months before entry, IFN was discontinued as a result of an increase of transaminases x 3 followed by rapid normalization of liver function tests and progressive re-introduction of IFN. In a subsequent routine liver test 1 month later, he presented alkaline phosphatase 26 UI/dL, AST 774 UI/dL, ALT 1,280 UI/dL (Table 2). Antithyroperoxidase antibodies were positive and TSH was 7.2 uIU/mL. IFN was discontinued and thyroid supplementation started with levothyroxine 100 μgr/day. One month later, AST was 620 UI/dL, ALT 480 UI/dL, alkaline phosphatase 64 UI/dL and prothrombin time was 51 %, refractory to vitamin K parenteral supplementation. The patient had no history of previous hepatic disease, no alcohol consumption or use of other hepatotoxic drugs. Pre-treatment liver biochemistry was normal. Viral serology for hepatitis A, B and C were negative. One week later, transaminases were AST 1,096 UI/dL, ALT 2,209 UI/dL. ANA titer was 1/320 (speckeled pattern), with negative anti smooth muscle and antimitochondrial antibodies. Gammaglobulin level was 2.8 mg/dL. Prothrombin time fell to 42%, with factor V 48 % without evidence of hepatic encephalopathy. A transjugular liver biopsy was performed, that demonstrated severe periportal interface hepatitis with plasma cell infiltration, piecemeal necrosis and bridging, without evidences of fibrosis.
Laboratory data of case 2.
| Case 2 | - 4 months | - 5 weeks | - 3 weeks | -2 weeks | Admission | + 15 days |
|---|---|---|---|---|---|---|
| Hb (gr/dL)/ White cell count (milli/mm3) | 14.2/ 7,200 | 13.9/ 7,980 | 13.9/ 8,130 | 13.8/ 8,900 | 12.9/ 9,560 | 13.8/ 9,820 |
| Glycemia (mg/dL) | 91 | 89 | 86 | 78 | 84 | 99 |
| Creatinine (mg/dL) | 0.8 | 0.5 | 0.7 | 0.6 | 0.6 | 1.1 |
| Total /Dir Bil (mg/dL) | 0.7/0.4 | 0.6/0.4 | 1.9/ 1,5 | 3.4/ 2.7 | 3.2/ 2.9 | 1.2/0.9 |
| AST/ALT (< 40 UI/L) | 96/105 | 774/ 1,280 | 620/970 | 1,096/ 2,209 | 1,360/ 2,450 | 160/204 |
| Alk Phosph (< 100 UI/L) | 52 | 26 | 64 | 101 | 100 | 79 |
| GGT (< 49 UI/L) | 38 | 28 | 54 | 69 | 78 | 62 |
| Quick time (%) | 80 % | 84 % | 81 % | 42 % | 39 % | 69 % |
| Factor V (%) | - | - | - | 48 % | 45 % | 81 % |
| Gammaglob (gr/dL) | - | - | 2.1 | 2.8 | 2.6 | 1.7 |
The patient started treatment with 60 mg/day of prednisone, followed by dose reduction by 15 days post-treatment He presented progressive normalization of transaminases and a rapid recovery of liver synthetic parameters (Figure 2). Azathioprine was started on day 20 post-steroids. The patient remained asymptomatic, with normal liver function tests. He denied a new liver biopsy, and was weaned from immunosuppression 3 years post-treatment. He presented no relapse post steroids and AZA removal. MS remained stable, without any further relapses by three years of follow up.
DiscussionIt has been reported that both type I IFNs (alpha and beta) share their side effects profile, though it has been speculated that their different receptor binding affinity and bioavailability may result in different side effects severity.1,6 Their most frequent untoward effect is an acute influenza like syndrome beginning several hours after injection. Dose limiting toxicities are bone marrow suppression, neurotoxicity, asthenia, depression, thyroid dysfunction and cardiotoxicity.1
Liver abnormalities during IFN therapy are usually asymptomatic and restricted to transient biochemical events.7 Yet, it has been observed that IFN alpha may induce hepatic decompensation and liver failure, especially in cirrhotic patients with chronic hepatitis B or C.8–10 Possible explanations have been related to exacerbations by IFN of immune mediated lysis of virus-infected hepatocytes,10 direct cytotoxic effect8 or induction of autoimmune events.11,12 It has also been observed the development and/or exacerbation5,13 of AIH in association with IFN alfa treatment for hepatitis C, a viral infection that per se has been related with autoimmunity.14–16
Multiple sclerosis is a demyelinating disease of autoimmune nature resulting from dysregulation of TH1 and TH2 lymphocytes, cytokines and regulatory T cells. MS patients have a higher level of responsiveness to autoantigens compared to controls, yet autoimmune events induced by IFN beta have been usually restricted to the production of autoantibodies, with the thyroid being the most common target organ involved.17,18 Overall incidence of thyroid dysfunction has been observed in 33% over 1 year of IFN beta therapy17 and is usually subclinical and transient.18–22
Exacerbations of autoimmune diseases, though unusual, have also been described, including Myasthenia gravis, Raynaud’s phenomenon, sarcoidosis, rheumatoid arthritis and lupus erythematosous. Yet, information related to the potential of IFN beta in multiple sclerosis patients to induce or exacerbate autoimmune liver disease is limited and controversial.
Liver involvement among patients with MS receiving IFN Beta is limited to non specific increases of citolytic hepatic enzymes. These abnormalities have been observed in about 20% of patients in the IFN beta 1b trial in MS and in about 30% of patients in post marketing surveillance follow up studies.23,24
These changes were seldom serious and over 50% occurred during the first three months of exposure to IFN beta, while only 11 % persisted after two years post treatment.25
Different reports have also described the development of autoantibodies related to autoimmune liver disease, mainly antinuclear antibody and anti smooth muscle antibody.
Verdun demonstrated the development de novo of autoantibodies in 7.2 % of patients. Yet, he did not observe any correlation between the positivity of autoantibodies at baseline or during treatment and the occurrence of any liver function test alteration.26 Speciale observed that both ANA and ASMA may fluctuate over time during treatment without evidence of clinical impact, except for one patient in their prospective series of 69 patients in whom IFN treatment had to be interrupted after 6 months because of the occurrence of high ASMA levels associated with typical manifestations of autoimmune hepatitis, yet with no evidences of liver failure.27
Minor liver function tests alterations have also been described in patients with MS independently of treatment with IFN. The association of MS with AIH has been described with a prevalence of 0.17% within a French cohort of MS compared to 0.0016 in the general population.28 Durelli observed liver alterations in 4.6% of patients with MS at baseline, stepping to 37.5% after initiation of IFN beta treatment.20
Whether significant liver abnormalities with higher transaminases levels and associated autoantibodies represent true autoimmune hepatitis is controversial.
Several reports have emphasized the development of compatible clinical, serological and pathological pictures of autoimmune hepatitis among these patients, yet in some of these descriptions clinical condition improved with the discontinuation of IFN, without requirement of steroids or other immunosuppressive medications. It can also be discussed if this is a real association or a coincidental presentation of two autoimmune diseases in a predisposed individual, maybe favored by the concurrent use of IFN.
Though rare, severe cases of autoimmune hepatitis have already been described in this group of patients. Two reports of liver failure complicating the treatment of MS have been published,29,30 yet in one of them requiring emergency liver transplantation information obtained after its publication revealed that the patient had received a known hepatotoxic medication, nefazadone, that has been associated with severe liver failure in more than 20 patients and hepatic adverse effects in 109 more.31,32
Considering this potential of IFN for considerable hepatotoxicity, limits of tolerable liver function laboratory abnormalities under therapy with IFN beta have been proposed and should be closely monitored, particularly during the first year of treatment or after re-trial. They include elevated total bilirrubin 2-3 times above normal values, transaminases or alkaline phosphatase over per 3, or the simultaneous increase of the three parameters. The finding of these alterations is an indication of dosage reduction. When extreme deviations of normal values occur, IFN treatment should be discontinued. In most instances hepatic enzyme abnormalities return to normal after discontinuation of therapy. Once these parameters normalize, it has been recommended that IFN beta can be reintroduced at 25% of the usual dose with a slow dosage building as liver function is monitored. Evidences of compromised liver synthetic function (ie fall in prothrombin time, factor V below 30% or development of hepatic encephalopathy) should prompt the evaluation of these patients by a Liver Transplantation Unit.33,34
Both of our patients presented with severe synthetic derangement associated with transaminases over x 10 and bilirrubin above 10 mg/dL. They had no previous history of liver dysfunction and presented with a normal pre-treatment liver biochemistry. They both developed severe acute hepatitis with positive autoantibodies either at presentation or during the progression of disease, high levels of gammaglobulin and a fall of prothrombin time. One of the patients behaved as a subfulminant liver failure with development of hepatic encephalopathy within three weeks of jaundice, sub massive hepatocellular necrosis and required listing for liver transplantation.
Pathologic examination of hepatic biopsies presented with typical features of autoimmune hepatitis with no evidences suggestive of chronic hepatitis or significant fibrosis. Pre-treatment IHAG score35 for patient 1 was 13 (probable autoimmune hepatitis) and for patient 2 it was 16 (definitive autoimmune hepatitis), both responsive to steroids. IFN discontinuation and rapid initiation of corticosteroid treatment led to full recovery in both patients, with rapid normalization of synthetic function. It can be argued that the usefulness of immunosuppressive therapy in severe and fulminant forms of AIH has not been fully demonstrated. Moreover, the fact that corticosteroid therapy might obviate the need for liver transplantation (LT) has not been proven.36 Yet, in our own experience,37 and as it has been reported in diverse reports, the early introduction of steroids can control acute inflammation and allow the recovery of liver function.38,39 Whether these cases represent simply hepatotoxicity or IFN behaves as a trigger unmasking AIH in a predisposed individual is difficult to define. Yet, management with drug discontinuation and immunosuppression seems to be above discussion.
As far as we know, this is the first report of severe autoimmune hepatitis developing years after initial exposure to IFN beta 1a therapy.
It should be kept in mind that although liver enzyme elevations during IFN treatment in patients with multiple sclerosis are usually mild and transient, they could hallmark the development of severe, life threatening autoimmune hepatitis, and even after IFN discontinuation evolve to liver failure if untreated. Rapid evaluation with liver biopsy and prompt initiation of corticosteroids may prevent the progression of liver failure and the need for liver transplantation.