Intrahepatic cholangiocarcinoma (ICCA) comprises 10% of all cholangiocarcinoma (CCA). It can be divided into three macroscopic subtypes, the least common of which is characterized by intraductal growth and believed to be more amenable to good outcomes with surgical resection compared to other ICCA. Recently, the rare finding of oncocytic differentiation has been described in this subtype and termed «intraductal oncocytic papillary neoplasm» (IOPN), but it remains unclear if the presence of oncocytes confers a different tumor behavior. We present the eighth reported case of IOPN, which to our knowledge, is the first such case that, due to its location and vascular compromise, required orthotopic liver transplantation (OLT). This case adds to the little that is known about the behavior of IOPN and supports the observation that resection, or OLT when resection is not possible, is a valid treatment option.
Cholangiocarcinoma (CCA) is the second most common neoplasm of the human liver after hepatoma.1-4Intrahepatic cholangiocarcinoma (ICCA) is a primary adenocarcinoma arising from the epithelium of the second or more distal branches of the intrahepatic ducts and is hence also termed peripheral CCA.4-6 It comprises approximately 10% of all CCA, while the remaining 90% originate from the extrahepatic or hilar ducts.4,6-8
Surgical resection is the primary treatment for ICCA.7,9 Resectable ICCAs are divided into 3 fundamental macroscopic subtypes: 1) mass-forming, 2) periductal-infiltrating, and 3) intraductal growth.10,11 Intraductal ICCA is the least common of these subtypes, comprising only 8-14% of all ICCA cases.10-12 It is characterized by papillary growth in the bile duct lumen with no or micro-invasion beyond the bile duct walls, and is believed to be analogous to intraductal papillary mucinous neoplasms of the pancreas (IPMN).13-16 The few available reports on intraductal ICCA have suggested less aggressive tumor behavior and the potential for better outcome with resection compared to other ICCAs despite the typically large tumor size (> 5 cm) at presentation.17 In 2002, the finding of oncocytic differentiation was described as a variant of this uncommon tumor subtype; it remains to be seen if the presence of oncocytes alters the course of disease.17
We present a patient who was diagnosed with a large intraductal ICCA with the rare finding of oncocytic differentiation, hence an intraductal oncocytic papillary neoplasm (IOPN) of the liver. This is the first case, to our knowledge, of bilobar tumor which was treated with orthotopic liver transplantation (OLT). We hope this case will add to the little known about intraductal ICCA, and more specifically, IOPN.
Case reportThe patient is a 50-year-old Japanese-American female who presented to her primary care physician with several days of progressive weakness, anorexia, nonproductive cough, right shoulder and pleuritic chest pain, and lower extremity edema. She was a non-smoker, non-drinker with no past medical history except Grave’s disease which was treated with thyroid radioablation 15 years prior. An outpatient chest X-ray revealed bilateral foci of opacification, while the computerized tomography (CT) of the chest and abdomen revealed extensive enhancing liver lesions, the largest involving the left and right lobes and measuring 10.3 cm in greatest dimension, large air fluid levels in the right lobe of the liver, left portal vein and right and middle hepatic vein thrombi extending into the inferior vena cava, multiple pulmonary nodules, a large lingular mass with air fluid levels, and moderate right-sided pleural effusion. The patient was scheduled for liver biopsy to aid in diagnosis, but her condition rapidly deteriorated to where she could not care for herself anymore, thus requiring hospitalization.
She was an alert and oriented, thin women in mild respiratory distress. Vital signs were blood pressure 120/80 mm/Hg, pulse 90 beats per minute, temperature 36.5 °C, respiratory rate 20 breaths per minute, and arterial oxygen saturation 95% on 2 L of oxygen by nasal cannula. Head and neck examination was unremarkable, including absence of lymphadenopathy. Cardiopulmonary examination was significant for decreased breath sounds over the lower lung fields, particularly the right. The abdomen was somewhat distended and tympanitic, but without tenderness, rebound, or hepatosplenomegaly. The remainder of the physical examination was unremarkable, except for 3+ pitting edema involving the bilateral lower extremities. Pertinent laboratory results included white blood cells 38.0*103/μL with 81% neutrophils, hemoglobin 9.4 g/dL, and platelets 268*103/μcL. Chemistries were significant for a sodium of 131 mmol/L and creatinine of 1.2 mg/dL. Liver tests were remarkable for alanine aminotransferase of 49 U/L (normal 5-50 U/L) and alkaline phosphatase (ALK) of 484 U/L (normal 35110 U/L). International normalized ratio (INR) was 1.3 and partial thromboplastin time (PTT) was low at 22.4 (normal 24.6 to 32.4 seconds), and a hypercoagulability panel revealed an elevated homocysteine level at 13.1 μ mol/L (normal 3.3-10.2 μ mol/L) but negative Factor V R506Q mutation, Prothrombin G20210A mutation, and Antiphospholipid antibodies as well as normal Anti-thrombin III activity. Hepatitis A, B, and C serologies were negative. Tumor markers revealed normal Alpha-fetoprotein (AFP), Carcino-embryonic antigen (CEA), and Carbohydrate antigen 19-9 (CA19-9).
Given the patient’s clinical presentation and imaging data, she was empirically started on antibiotics as well as anticoagulation due to concern for possible pulmonary embolism (PE). On the day after admission, the patient underwent left lobe liver biopsy with placement of two drains. Purulent fluid was noted to be draining, and culture revealed viridans Streptococci. Based on the biopsy material, a diagnosis of «oncocytic papillary carcinoma of the biliary tract» was made. Given the clinical information, it was concluded that the patient had a primary intrahepatic biliary neoplasm complicated by infection and abscess formation, with consequent septic emboli to the lungs.
With continued antibiotic therapy and anticoagulation, the patient experienced some improvement in energy, appetite, cough, shoulder and pleuritic chest pain, and lower extremity edema. Repeat CT scan one week after admission revealed interval development of a cavitary lesion in the right upper lobe likely representing pneumonia or a septic embolus, a small left-sided pleural effusion, an increase in the size of the right-sided pleural effusion, and PE in the right upper lobe pulmonary artery. The lingular lesion and right lobe cavitary fluid-containing lesions were unchanged, as were the portal and hepatic venous defects (Figure 1a). The patient remained in the hospital for a total of 16 days, after which she was discharged home on subcutaneous enoxaparin injections and antibiotics through a peripherally-inserted central venous catheter, with hepatology, surgery, and infectious disease follow-up.
Abdominal magnetic resonance (MR) angiogram obtained as an outpatient showed multiple thrombosed venous vessels and a stenotic left hepatic vein. It was felt that the patient’s hepatic blood supply would be excessively compromised by performing a partial hepatectomy. Despite the large size of the tumor, recognizing its slow growth rate and the low likelihood of metastasis, the possibility of liver transplantation was raised.
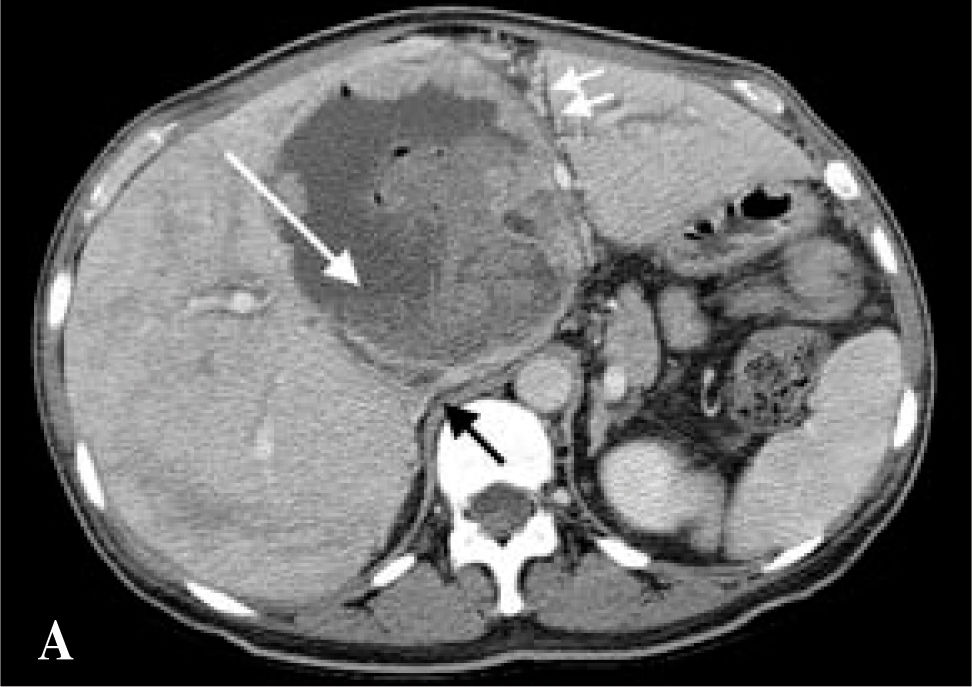
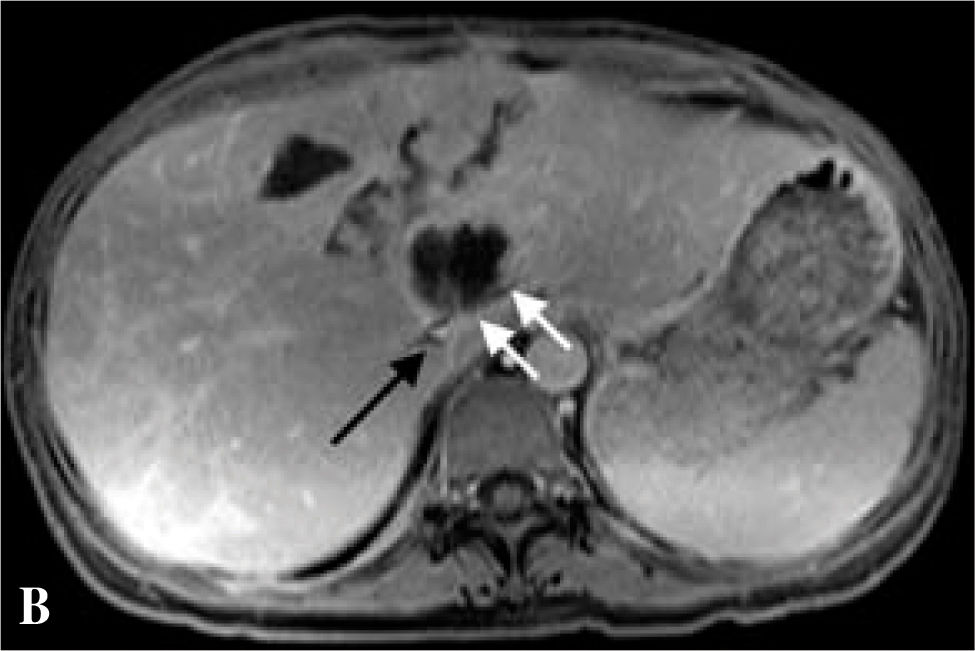
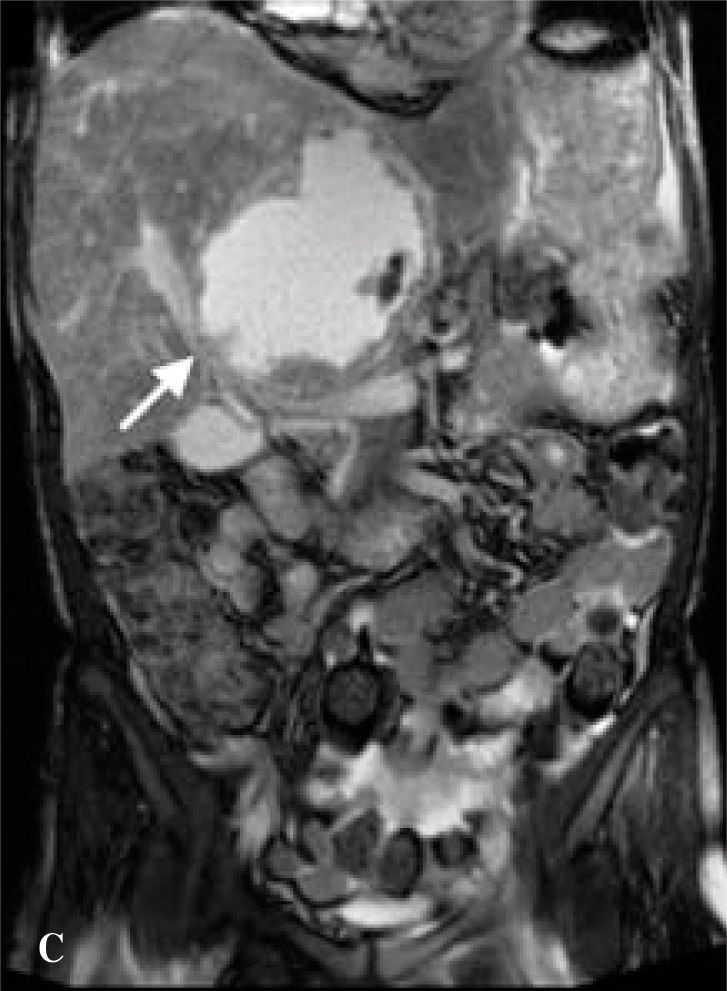
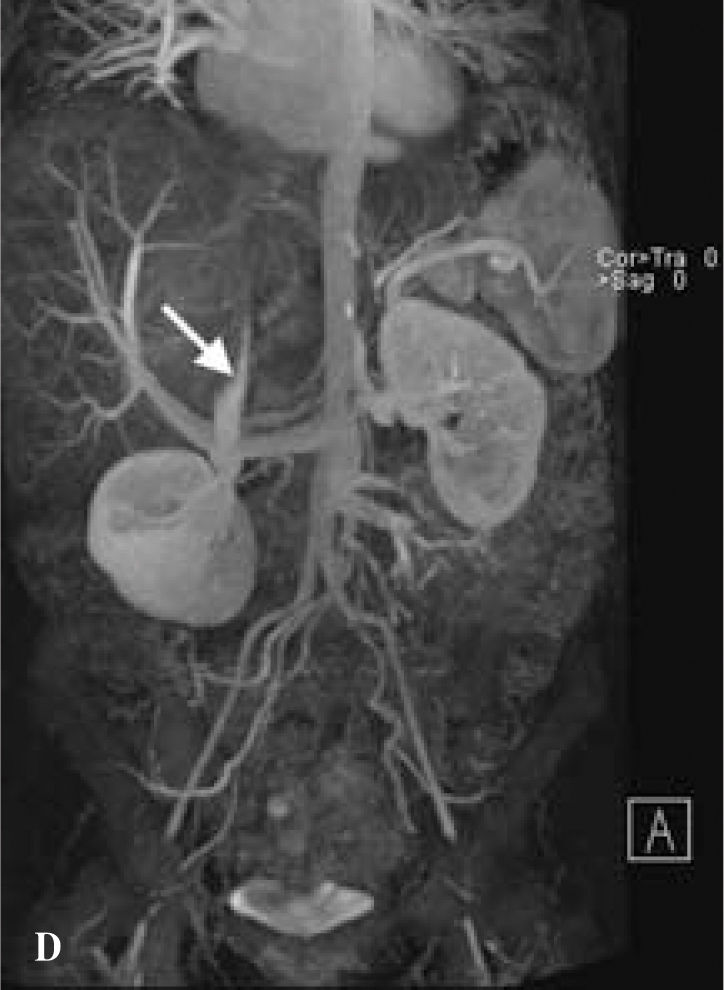
The patient was referred to our liver transplantation center. On presentation, she had normal vital signs and was in no acute distress. Physical examination was remarkably normal except for a large, non-tender mass in the right upper quadrant and epigastrium that moved with deep inspiration but did not produce a hepatic bruit. CBC complete blood count and chemistries were normal. Liver tests were remarkable only for (ALK) of 205 U/L. AFP, CEA, and CA-19-9 tumor markers were not elevated. The patient underwent MR of the abdomen with and without contrast, which revealed one enhancing cavitary mass measuring approximately 11.3 by 11.5 cm spanning the left and right lobes and resolution of the other liver lesions previously seen on CT. The right hepatic vein was thrombosed, the left hepatic vein was stenotic, the right portal vein was compressed, the left portal vein was either compressed by the mass or thrombosed, and there was a small persistent IVC thrombus, as on prior imaging (Figures 1b,1c,1d).
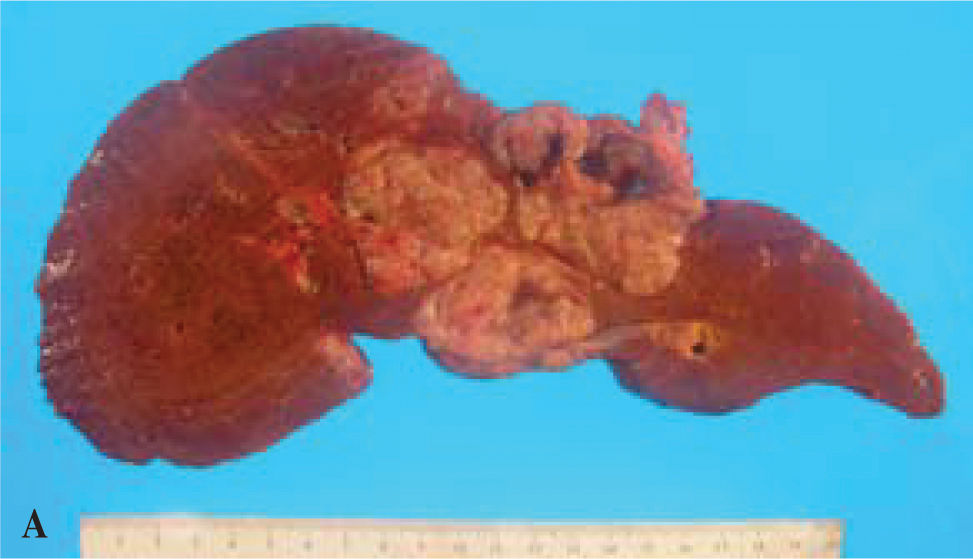
The patient was deemed an appropriate candidate and underwent OLT with choledochocholedochostomy and T-tube placement, as well as partial segmental resection of the second portion of the duodenum with primary closure. The latter was done because of tumor adherence to the duodenum. The patient’s post-operative course was complicated by acute renal failure which resolved without requiring dialysis. Gross examination of the specimen revealed an approximately 13 cm mass which obscured the porta hepatis (Figure 2a). The mass consisted of a number of closely-approximated, well-circumscribed nodules which appeared to be intraluminal. Histologic examination revealed dilated ducts filled with a papillary neoplasm composed of thin fibrous septae lined by multiple layers of cells punctuated by sharply-defined round lumens (Figure 2b). The cells were large and had abundant granular, esosinophilic cytoplasm characteristic of oncocytic differentiation (Figure 2b). The nuclei were round with mild to moderate atypia. The ducts were lined either by similar appearing cells or by flattened bile duct epithelial cells. Multiple sections of the interface between the tumor and the surrounding liver parenchyma demonstrated hyalinized stroma with small foci of microinvasive tumor. There was no extension into the adjacent liver parenchyma. Surrounding bile ducts were dilated and frequently contained neurophils and necrotic debris. Immunohistochemistry was performed, and the results, while nonspecific, were characteristic of intraductal oncocytic papillary neoplasm of the bile duct. The patient was discharged 18 days after OLT, and at 6-months post-OLT follow-up continues to do well, without recurrence of malignancy.
Low-power microscopic view of papillary tumor filling a bile duct lumen. The lumen is lined by both flattened, atrophic biliary eipthilum (white arrow) and by tumor cells (black arrow); inset showing high-power view of characteristic oncocytic cells with abundant, granular, eosinophilic cytoplasm.
Intraductal ICCA is the newest-described macroscopic subtype of ICCA.10,11 Several conditions involving chronic inflammation of the bile ducts are believed to predispose to ICCA, including PSC, hepatotropic trematodal infection, and hepatolithiasis, although most patients, including those with the intraductal subtype, have no identifiable risk factors.9,18 The average age at presentation is 55 years, and patients typically do not present until the disease is relatively advanced.9,18
Surgical resection, which is the primary treatment for ICCA, is indicated for patients without vascular invasion and extrahepatic metastasis and if the tumor is not too large to compromise liver function if resected, although post-operative outcome remains poor due to recurrence of disease, with median survival being only 3 years.7,9 Post-operative survival rates may, however, be more favorable in the cohort of patients with early-staged tumor, well-differentiated tumor, absence of physical findings, presence of mucobilia, negative histologic margins, and the intraductal ICCA sub-type.9,12,18,19
In 2002 Martin et al. reported 3 cases and reviewed another 39 cases of intraductal ICCA reported in the world literature. Since then, there have been several more case reports and series with a total of over 200 patients.13,15,20-26 In the cases where there was sufficient information, follow-up was usually on the order of 1-3 years, and nearly all patients were doing well and disease-free at the time of follow up. Given the encouraging survival rates, it has been hypothesized that the intraducatal subtype of ICCA has good prognostic implications over the mass-forming & periductal-infiltrating subtypes.16,27
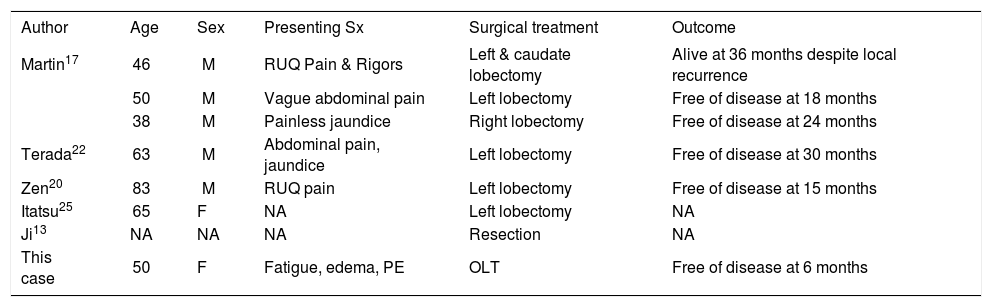
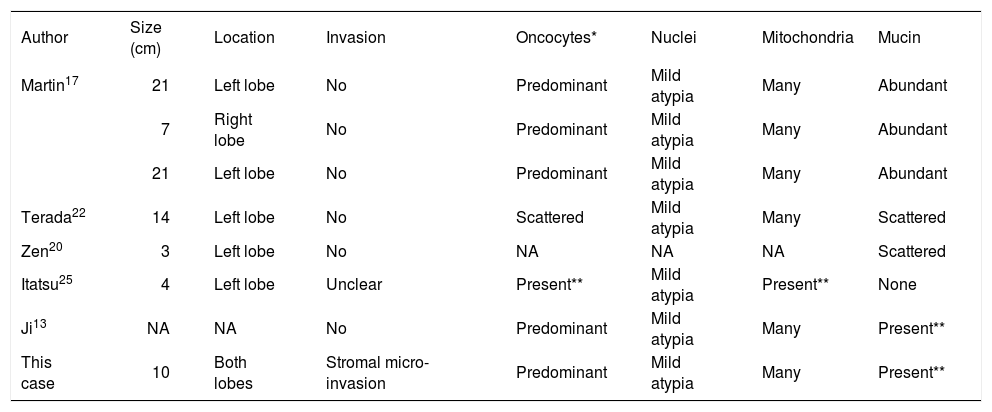
Comprising only a small fraction of intraductal ICCA is the variant containing oncocytic differentiation, IOPN. The term oncocyte was coined in 1931 by Hamperl, referring to epithelial cells with abundant, brightly eosinophilic and granular cytoplasm, and high numbers of mitochondria.28 These cells occur in a variety of organs, both in benign as well as malignant tumors.28,29 Oncocytic differentiation in the biliary tract was first described by Wolf et al. in 1992 in a cystadenocarcinoma.30 The first report of IOPN was not until 2002, and since then only 7 of all the cases of intraductal ICCA in the English language literature were of the IOPN variant.13,17,20,22,25 Our patient represents the 8th known case with this rare histologic finding. A synopsis of all 8 cases in chronological order is provided in tables 1a and 1b.
Patient data.
| Author | Age | Sex | Presenting Sx | Surgical treatment | Outcome |
|---|---|---|---|---|---|
| Martin17 | 46 | M | RUQ Pain & Rigors | Left & caudate lobectomy | Alive at 36 months despite local recurrence |
| 50 | M | Vague abdominal pain | Left lobectomy | Free of disease at 18 months | |
| 38 | M | Painless jaundice | Right lobectomy | Free of disease at 24 months | |
| Terada22 | 63 | M | Abdominal pain, jaundice | Left lobectomy | Free of disease at 30 months |
| Zen20 | 83 | M | RUQ pain | Left lobectomy | Free of disease at 15 months |
| Itatsu25 | 65 | F | NA | Left lobectomy | NA |
| Ji13 | NA | NA | NA | Resection | NA |
| This case | 50 | F | Fatigue, edema, PE | OLT | Free of disease at 6 months |
Tumor data.
| Author | Size (cm) | Location | Invasion | Oncocytes* | Nuclei | Mitochondria | Mucin |
|---|---|---|---|---|---|---|---|
| Martin17 | 21 | Left lobe | No | Predominant | Mild atypia | Many | Abundant |
| 7 | Right lobe | No | Predominant | Mild atypia | Many | Abundant | |
| 21 | Left lobe | No | Predominant | Mild atypia | Many | Abundant | |
| Terada22 | 14 | Left lobe | No | Scattered | Mild atypia | Many | Scattered |
| Zen20 | 3 | Left lobe | No | NA | NA | NA | Scattered |
| Itatsu25 | 4 | Left lobe | Unclear | Present** | Mild atypia | Present** | None |
| Ji13 | NA | NA | No | Predominant | Mild atypia | Many | Present** |
| This case | 10 | Both lobes | Stromal micro-invasion | Predominant | Mild atypia | Many | Present** |
Although there are few cases and insufficient follow up from which to draw firm conclusions, it appears there is little, if any, clinical significance to oncoyctic differentiation in intraductal ICCA. The biologic behavior and prognosis of IOPN seems similar to that of intraductal ICCA, in whom the 5-year survival after resection is near 80%.16,27 In our case, because resection was not an option, OLT was performed. This has not been done previously in cases of IOPN, but was deemed appropriate in our patient given the encouraging results with resection in the literature and the patient’s overall wellbeing. At her 6-month post-operative follow-up appointment, the patient was feeling well and had no evidence of tumor recurrence. Her long term outcome remains to be seen, although based on previous reports, it is expected to be favorable.
In conclusion, intraductal ICCA is an uncommon subtype of cholangiocarcinoma that includes a rare variant, IOPN. Similar to intraductal ICCA without oncocytic differentiation, long-term survival or cure may be achieved with complete resection of IOPN. In cases where resection is not possible due to tumor size or bilobar tumor, we believe OLT is a valid option given the slow growth and minimal metastasis of IOPN. Accumulation of future data on this variant will help further characterize its clinical features, treatment, and prognosis.