Recent advances have been achieved in the management of hepatitis C. However, information regarding epidemiology, natural history, and short and long term patient outcomes in Latin America are scarce. Even more there is no clear consensus among infectious diseases, gastroenterology, hepatology, and virology experts on our geographic area and there are relevant differences in each country regarding chronic hepatitis C patient’s management. This encouraged the organization of a Latin American Consensus Conference in order to review current knowledge of Hepatitis C infection.
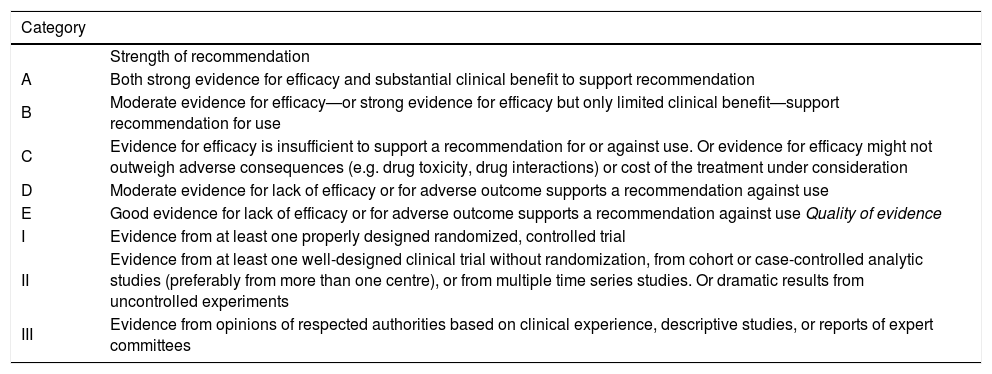
This meeting included fifty five basic and clinical experts from fourteen Latin American countries. An organizing committee drafted 100 questions to be addressed during a two days conference, that was divided in 20 modules that approached controversial issues on Hepatitis C infection. After each presentation there was an open discussion in which each recommendation and quality of evidence were decided by direct voting. A consensus was considered when more than 60% of attendees agreed with the statement. The statements and recommendations were graded for their quality according to the Infectious Disease Society of America (IDSA) System (Table).
Grading scheme for recommendations.
| Category | |
|---|---|
| Strength of recommendation | |
| A | Both strong evidence for efficacy and substantial clinical benefit to support recommendation |
| B | Moderate evidence for efficacy—or strong evidence for efficacy but only limited clinical benefit—support recommendation for use |
| C | Evidence for efficacy is insufficient to support a recommendation for or against use. Or evidence for efficacy might not outweigh adverse consequences (e.g. drug toxicity, drug interactions) or cost of the treatment under consideration |
| D | Moderate evidence for lack of efficacy or for adverse outcome supports a recommendation against use |
| E | Good evidence for lack of efficacy or for adverse outcome supports a recommendation against use Quality of evidence |
| I | Evidence from at least one properly designed randomized, controlled trial |
| II | Evidence from at least one well-designed clinical trial without randomization, from cohort or case-controlled analytic studies (preferably from more than one centre), or from multiple time series studies. Or dramatic results from uncontrolled experiments |
| III | Evidence from opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees |