Background. Current practice guidelines recommend liver biopsy prior to treatment of hepatitis C genotype-1 but not for genotype-2/3; this is based on expert opinion, not on published evidence. Methods. In retrospective analysis of a large trial database prior to the publication of recent guidelines, we compared outcomes in 985 treatment-naïve patients with hepatitis C who did or did not undergo liver biopsy before starting peginterferon alfa-2a plus ribavirin. Results. Physicians elected to treat 141/654 (21.6%) genotype-1 patients and 126/331 (38.1%) genotype-2/3 patients without liver biopsy. There were no differences in baseline characteristics among those with or without pre-treatment liver biopsy, except for female preponderance in genotype-1 patients with liver biopsy. The sustained viral response (SVR) rate was no different amongst genotype-2/3 patients who had a biopsy before treatment with 66.3% SVR vs. 69.8% of those treated without biopsy (p = 0.546), but significantly higher among genotype-1 patients with pre-treatment liver biopsy at 54.6 vs. 44.0% for those treated without a liver biopsy (p = 0.029). In genotype-1 patients with liver biopsy, more patients with cirrhosis had dose adjustments (p = 0.0057) rather than drug discontinuation. There was tendency for earlier discontinuation among patients without pre-treatment liver biopsy. Conclusions. Pre-treatment liver biopsy was associated with better SVR amongst genotype-1 patients. This improvement may reflect ongoing commitment to completing the treatment course by both patient and physician. In genotype-2/3 patients, pre-treatment liver biopsy may not be essential to maximize SVR rates. This study validates the recommendations of the most recent treatment guidelines for hepatitis C.
Liver biopsy has been the cornerstone for the diagnosis and management of liver disease. Over the past two decades, however, new tests and procedures have increased our diagnostic ability. In particular for liver disease due to chronic viral infections, virological and biochemical assays, and ultrasonographic examination of the abdomen have reduced the uncertainty of clinical diagnosis, with progressively greater efficiency and lower risk.1-4 Since the level of certainty needed in decision making is a function of the characteristics of available therapies, one may tolerate substantial diagnostic uncertainty if the treatment cures the disease.5-7
As progress in antiviral therapy against hepatitis C virus (HCV) infection is made,8,9 information obtainable from liver biopsy may have less influence on treatment decisions. Based on these premises, the value of a procedure with well-known risks10,11 should be critically re-examined. Because the decision to perform a liver biopsy is firmly rooted in physicians’ preference, past expert consensus guide-lines have recommended the routine performance of liver biopsy before initiation of antiviral therapy for chronic HCV.12-14
More recent guidelines have shifted the emphasis suggesting that liver biopsy may not be necessary for genotype-2/3 but still recommending it in patients infected with HCV genotype-1 if treatment is being considered. These recommendations have been based on expert-opinion rather than published evidence.
Patients are not always eager to have a liver biopsy, and frequently have anticipatory anxiety, which would be expected of a procedure that is associated with complications and death.10
Guidelines are commonly issued by scientific committees and often do not incorporate patients’ preference into medical decision making. Moreover, liver biopsy may not always be representative for stage or grade of the liver disease, as sample bias, and interor intra-observer variability affect the diagnostic yield.15-18
A cost-effectiveness analysis suggested that the best strategy in the management of patients with HCV infection is to offer therapy to all patients and not to biopsy,19,20 and that the histopathological diagnosis is of little additional value for the recommendation of treatment16 empirical treatment based on HCV-RNA quantification, or HCV genotyping but without liver biopsy, has acceptable cost-effectiveness.19
These conclusions, derived from mathematical simulation based on Markov computer modeling, require direct clinical verification before becoming widely accepted. At minimum, patients would like to see some evidence that a liver biopsy would make a difference in outcomes before accepting the expert advice. Physicians who treat HCV also would like to see that in genotype-2/3 the decision for initiating therapy in patients without a pre-treatment liver biopsy would not impact the outcomes.
In this study, we compared sustained viral response (SVR) rates after combination treatment with peginterferon alfa-2a and ribavirin in treatment-naïve patients with HCV infection who underwent or did not undergo liver biopsy prior to initiation of therapy.
Material and MethodsPatientsPatients included in this retrospective analysis of prospectively collected data were enrolled in the open-label Canadian Pegasys Expanded Access Program (EAP). Eligibility criteria for the program were broad and previously described.21 Individuals were required to have a clinical diagnosis of chronic HCV and quantifiable HCV RNA in serum. Patients with clinically advanced liver disease could be enrolled only if they had compensated liver disease (Child-Pugh class A), neutrophil count > 1.5 x 109/L, and platelet count > 90 x 109/L. Only treatment-naïve Only treatmentna in this analysis. Also all patients with multiple genotypes or genotype-4/5/6 were excluded.
Liver biopsyIn the second phase of EAP, there was no requirement for a liver biopsy to enroll patients. The decision was left to the usual clinical practice of the participating physician. Any biopsies performed on patients enrolled in the EAP were read by the presiding local pathologist at each study site. For those who underwent liver biopsy, grading of inflammation and staging of fibrosis were analyzed according to the METAVIR scoring system. Only the most recent biopsy result was recorded on the case report form. For patients who were treated without a liver biopsy, the reason for not performing a liver biopsy was not collected on the case report forms.
TreatmentPatients received peginterferon alfa-2a 180 μg/week plus either a low fixed dose of ribavirin (800 mg/day) or a weight-based dose of ribavirin (1,000 mg/day for patients with a body weight < 75 kg; 1,200 mg/day for patients with a body weight ≥ 75 kg) for 24 or 48 weeks (Pegasys RBV®, Roche, Mississauga, Canada).
The EAP was not a randomized trial; the duration of treatment and ribavirin dose were selected at the investigator’s discretion. The impact of differences in dosage has also been reported previously.22 In this analysis, only genotype-1 patients who were assigned to a ribavirin dose of 1,000/1,200 mg/day and planned treatment duration of 48 weeks were included. Among genotype-2/3 patients, only those assigned to a ribavirin dose of 800 mg/day and planned treatment duration of 24 weeks were included.
Limiting the study to patients who received optimum dosing and length of therapy was necessary to avoid baseline treatment biases and allow an intention-to-treat analysis.
OutcomesThe primary efficacy outcome was SVR, defined as undetectable HCV RNA (< 50 IU/mL by qualitative PCR assay, Cobas Amplicor HCV Test, v2.0, Roche Diagnostics, Indianapolis, IN, USA) 24 weeks following completion of therapy. Early virological response (EVR) was defined as 2-log10 or more drop in serum HCV RNA, or undetectable HCV RNA by quantitative PCR assay (Cobas Amplicor HCV Monitor, v2.0, Roche Diagnostics, Indianapolis, IN, USA; limit of quantification 600 IU/mL) at week 12. In genotype-1 patients those who had undetectable HCV RNA by qualitative PCR at week 24 continued treatment for total of 48 weeks.
End of treatment virological response (EOTVR) was defined as undetectable HCV RNA by qualitative PCR at the end of planned treatment (i.e., week 24 for genotypes-2/3 and 48 for genotype-1). Relapse rate was defined as percentage of patients with detectable HCV-RNA at 24 weeks post-treatment from those with undetectable HCV-RNA at the end of the treatment period.
Adverse events, dose adjustments and drug discontinuationSafety assessments (physical examination and laboratory evaluations) and monitoring for adverse events were conducted at regular intervals throughout EAP, entered into case report forms, and captured in the database.
In the event of clinically significant laboratory abnormalities or adverse events, the adjustment or discontinuation of dosage of peginterferon alpha-2a or ribavirin was left to the individual participating physicians who were all aware of the recommended guidelines. All these dose adjustments and drug discontinuations were also captured by the EAP case report forms.
Statistical analysisThe analysis for the current study was reported on the intention-to-treat approach. All patients registered in the second phase of EAP who received at least one dose of peginterferon alfa-2a and ribavirin were included. They were grouped either having liver biopsy or not having liver biopsy prior to treatment. Differences between groups were evaluated by Fisher’s exact test or Kruskal-Wallis test.
For the dose adjustment and drug discontinuation analysis, those with pre-treatment liver biopsy were further stratified by the presence or absence of cirrhosis (METAVIR score 4).
Time to dose adjustment or drug discontinuation for peginterferon alfa-2a and ribavirin was evaluated by cumulative failure plot similar to a Kaplan Meier survival analysis with comparison between groups by log rank test.
Baseline factors predictive of SVR were identified by multiple logistic regression analysis using a backward elimination process. Factors considered in the initial model included biopsy status (yes vs. no), age (per 10 year increment), sex (male vs. female), race (Caucasian vs. non-Caucasian), BMI (per unit increase), HCV RNA level (per log10 increment) and platelet count (< 150 vs. ≥ 150 x 109/L). All these factors were identified a priori since they are known to impact SVR.21
The α-level of significance for a two-tailed test was considered to be at p of 0.05. All statistical analyses were performed using SAS v. 9.1 (SAS Institute, Cary, NC, USA).
ResultsA total of 2,702 patients were originally enrolled into EAP at 72 centers in Canada. The first patient was enrolled in October 2001. A total of 1,620 patients were enrolled in the second phase of the trial when a liver biopsy was not a prerequisite. There were 985 treatment-naïve patients available for this analysis including 654 genotype-1 patients assigned to a ribavirin dose of 1,000/1,200 mg/day and treatment duration of 48 weeks, and 331 genotype-2/3 patients assigned to a ribavirin dose of 800 mg/day and treatment duration of 24 weeks. The baseline characteristics of these patients are presented intable 1.
Baseline characteristics of treatment-naïve patients included in the analysis by genotype and biopsy status.
| Characteristic | HCV genotype-1* (N=654) | HCV genotype-2/3† (N=321) | ||||
|---|---|---|---|---|---|---|
| Biopsy (n = 513) | No biopsy (n = 141) | P value‡ | Biopsy (n = 205) | No biopsy (n = 126) | P value‡ | |
| Age in yr | 47.3 ± 8.8 | 45.9 ± 9.0 | 0.144 | 44.8 ± 9.4 | 44.1 ± 9.8 | 0.368 |
| Male gender, n (%) | 346 (67.4) | 112 (79.4) | 0.007 | 125 (61.0) | 77 (61.1) | 1.0 |
| Race, n (%) | ||||||
| Caucasian | 420 (81.9) | 120 (85.1) | 0.602 | 143 (69.8) | 98 (77.8) | 0.041 |
| Asian/Pacific Islander | 49 (9.6) | 15 (10.6) | 18 (8.8) | 14 (11.1) | ||
| All other | 44 (8.6) | 6 (4.3) | 44 (21.5) | 14 11.1) | ||
| BMI in kg/m2 | 27.4 ± 4.8 | 27.3 ± 5.1 | 0.500 | 26.3 ± 4.7 | 27.3 ± 5.3 | 0.119 |
| Liver biopsy result (METAVIR fibrosis stage), n (%) | ||||||
| 0-1 | 142 (27.7) | NA | NA | 70 (34.2) | NA | NA |
| 2 | 190 (37.0) | - | - | 68 (33.2) | - | - |
| 3 | 94 (18.3) | - | - | 36 (17.6) | - | - |
| 4 | 61 (11.9) | - | - | 23 (11.2) | - | - |
| Other/unknown | 26 (5.1) | - | - | 8 (3.9) | - | - |
| Neutrophil count x 109/L | 4.0 ± 4.4 | 4.5 ± 6.3 | 0.843 | 4.0 ± 2.6 | 3.7 ± 1.3 | 0.509 |
| Hemoglobin in g/L | 150 ± 13 | 151 ± 13 | 0.585 | 147 ± 14 | 148 ± 15 | 0.640 |
| Platelet count x 109/L | 211 ± 69 | 201 ± 58 | 0.313 | 212 ± 63 | 212 ± 63 | 0.904 |
| Platelet count <150 x 109/L, n (%) | 69 (13.5) | 26 (18.4) | 0.139 | 35 (17.1) | 23 (18.3) | 0.882 |
| Serum HCV RNA level, log10 IU/mL | 6.01 ± 0.76 | 5.99 ± 0.69 | 0.448 | 5.99 ± 0.79 | 5.84 ± 0.83 | 0.127 |
NA: Not applicable. BMI: Body mass index. All values shown as mean ± SD (standard deviation).
All genotype-1 patients included in this analysis were assigned to receive a ribavirin dose of 1,000/1,200 mg/day and planned treatment duration of 48 weeks.
Participating physicians elected to initiate therapy for HCV without a pre-treatment liver biopsy in 141 (21.6%) genotype-1 infected patients and 126 (38.1%) genotype-2/3 patients. Amongst the 513 genotype-1 infected patients and 205 genotype-2/3, the staging of fibrosis according to METAVIR score were comparable with 11.9% of genotype-1 patients and 11.2% genotype-2/3 patients having cirrhosis (METAVIR score 4).
In genotype-1 patients, except for female preponderance in those with liver biopsy (32.6 vs. 20.6%, p = 0.007), there were no differences in baseline characteristics between the two groups. Female HCV patients may have lower liver enzymes and slower progressing liver disease both reasons for considering liver biopsy but this database did not allow further analysis. The ethnic distribution amongst genotype-2/3 patients between the two groups were different (p = 0.041) with less Asians or Pacific Islanders (8.8 vs. 11.1%) having undergone pre-therapy liver biopsy. The overall number of African-Canadians in this population was only 0.9%.
Specifically, for genotype-1, when considering the baseline variables that usually predicted lower SVR such as higher viral load, older age, higher BMI or lower platelet counts (reflecting more advanced liver disease and portal hypertension), no significant differences could be identified between the group who had pre-treatment liver biopsy versus the group who did not undergo liver biopsy prior to initiation of therapy.
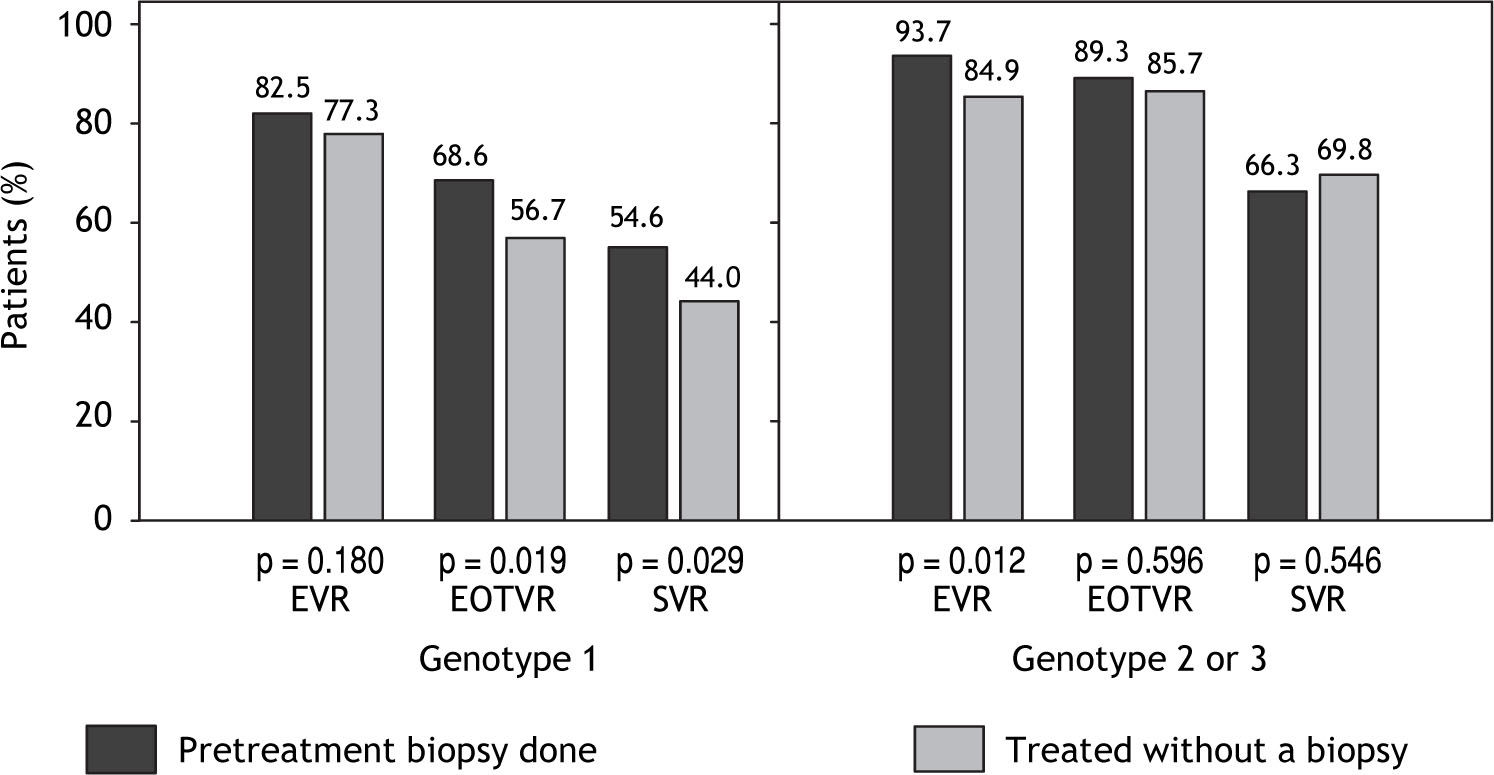
Virological responseEVR rate at week 12, EOTVR rate and SVR rate at 24 weeks following the completion of treatment are shown infigure 1. There was no significant difference in the proportion of genotype-2/3 patients treated without a biopsy who achieved an SVR (66.3%) when compared with patients treated without a biopsy (69.8%, p = 0.546).
Virological response rates at week 12 (EVR) the end of treatment (EOTVR) and after 24 weeks of following completion of treatment (SVR). When comparing with patients who were treated without a biopsy, those who had pre-treatment liver biopsy achieved 21.0% higher EOTVR and 24.1% higher SVR.
In contrast to the findings in genotype-2/3, 54.6% of those with a pre-therapy biopsy achieved an SVR, compared with 44.0% of those treated without a biopsy (p = 0.029); this unexpected 10.6% absolute improvement in SVR reflected 24.1% increase in SVR in those patients whose fibrosis stage was known before starting therapy. Further analysis revealed that after the first 12 weeks of treatment, there were no difference in EVR rates between the two groups among genotype-1 patients (p = 0.28), nor was there any difference in relapse rates with 20.4% in those with a pre-treatment biopsy vs. 22.4% in those treated without a biopsy (p = 0.687). The major difference and contributing factor to lower SVR was the EOTVR rate differences between the two groups (p = 0.019).
Safety and tolerabilityTo further elucidate the reasons for differences in EOTVR, we examined the number of dose adjustments and early discontinuation among the groups. There were no statistically significant differences in the frequency of ribavirin or peginterferon alfa-2a dose adjustment in patients infected with genotype-1 or genotypes-2/3 who were treated with and without a pre-treatment liver biopsy (Table 2) though there was tendency for more peginterferon alfa-2a discontinuation in the group that did not have pre-therapy biopsy; this did not achieve 2-tailed significance (p = 0.0632). Overall the incidence of non-serious adverse events was similar in genotype-1 patients and genotype-2/3 patients who were treated with a biopsy vs. those patients without a biopsy (Table 2).
Dose adjustments and reported adverse events in treatment-naïve patients by genotype and biopsy status.
| Characteristic | HCV genotype-1 (n = 654) | HCV genotype-2/3 (n = 321) | ||||
|---|---|---|---|---|---|---|
| Dose adjustments, n (%) | Biopsy (n = 513) | No biopsy (n = 141) | P value | Biopsy (n =205) | No biopsy (n = 126) | P value |
| Peginterferon alfa-2a | 217(42.3) | 63(44.7) | 0.0632 | 69(33.7) | 49(38.9) | 0.347 |
| Ribavirin | 249(48.5) | 70(49.6) | 0.849 | 59(28.8) | 47(37.3) | 0.116 |
| Patients with adverse events, n (%) | 207(40.4) | 51(36.2) | 0.383 | 66(32.2) | 31(24.6) | 0.171 |
Among genotype-1 patients, there were no significant differences between the two groups in the mean overall length of therapy with either peginterferon alfa-2a (286 vs. 270 days in patients with and without a biopsy) or ribavirin (291 vs. 275 days, respectively). Similarly, there were no significant differences between the two groups in the mean overall length of therapy with either peginterferon alfa-2a (159 vs. 152 days, respectively) or ribavirin (165 vs. 158 days, respectively) among genotype-2/3 infected patients.
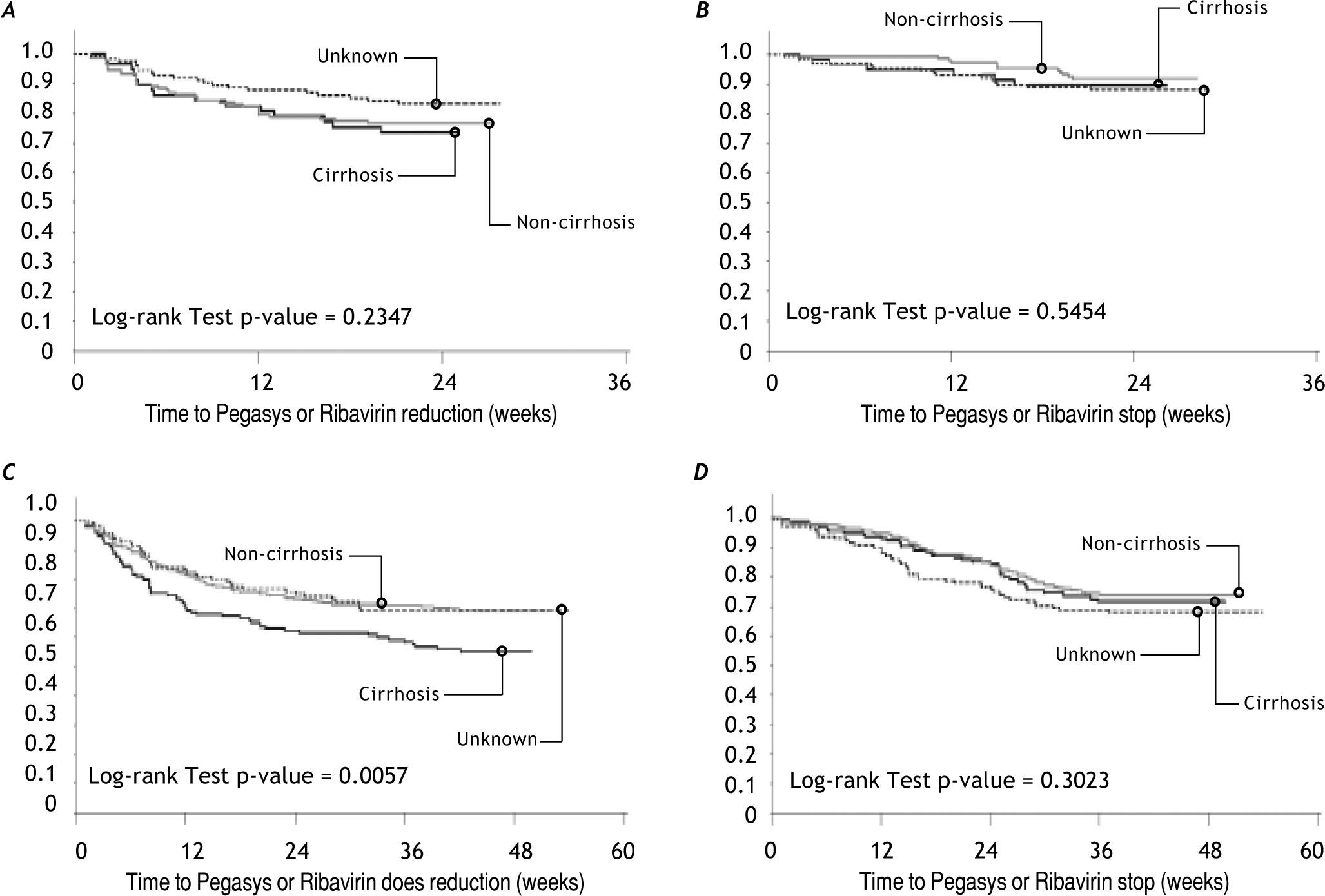
When the cumulative failure plots for dose adjustment and drug discontinuation were reviewed (Figure 2) for peginterferon alfa-2a or ribavirin in genotype-1 and genotype-2/3 patients, amongst those who underwent liver biopsy, more patients with cirrhosis had significantly more dose adjustments (p = 0.0057) especially during the first 12 weeks of therapy. The drug discontinuation was comparable (p = 0.3023) to those without cirrhosis or patients who did not undergo liver biopsy with discontinuations occurring around week-12 due to lack of early viral response or at week-24 because of ongoing HCV viremia by qualitative PCR testing. Also in genotype-1 patients who did not undergo liver biopsy prior to treatment, there was tendency for earlier drug discontinuation compared to patients who had undergone liver biopsy with or without cirrhosis.
In genotype-2/3 there were no differences in number of patients with dose adjustments in peginterferon alfa-2a or ribavirin (A) or drug discontinuation (B). In genotype-1 patients, there were significantly more dose adjustment (C) with p-value = 0.0057 rather than discontinuation peginterferon alfa-2a or ribavirin (D).
We proceeded by exploring the significant difference in SVR in genotype-1 patients. To avoid and correct for the inherent bias in the patient population, we conducted multiple logistic regression analysis to see if the availability of pre-treatment liver biopsy was independently associated with SVR. When baseline factors were entered into the stepwise analysis, the following were significantly associated with SVR in genotype-1 infected patients:
- •
Pre-therapy biopsy (OR 1.513 vs. no biopsy, 95% CI 1.024-2.234; p = 0.0376).
- •
Age (OR 0.827 per 10-year increment, 95% CI 0.685-0.998; p = 0.0473).
- •
Caucasian race (OR 0.435 vs. non-Caucasian race, 95% CI 0.279-0.678; p = 0.0002).
- •
HCV RNA level (OR 0.663 per 1-log increase, 95%CI 0.529-0.830; p = 0.0003).
- •
Platelet count level (OR 0.352 for < 150 vs. ≥ 150 x109/L, 95% CI 0.215-0.570; p < 0.0001).
Only age was significantly associated with SVR in the multiple logistic regression analysis of data from genotype-2/3 infected patients (OR 0.739 per 10-year increment in age, 95% CI 0.576-0.948; p = 0.0173).
DiscussionAll patients with chronic HCV are potential candidates for specific antiviral therapy. Originally, given the modest response to interferon monotherapy, treatment was recommended only in patients in whom the impact of treatment could be greatest, in terms of halting disease progression and preventing complications. On this premise early treatment guidelines recommended that decisions about treatment should be made only after performing a liver biopsy.12-14
However, in recent years we have witnessed a remarkable improvement in the clinical effectiveness of treatment of chronic HCV, with SVR rates of up to 50% in genotype1- patients and up to 80% in patients infected with genotype-2/3.8,9,22 Revision of guidelines would be expected to follow this therapeutic gain: as the probability of achieving an SVR increases, the need for absolute certainty in staging and grading chronic viral hepatitis decreases.
One would expect that a selective rather than routine liver biopsy policy would be used to assist in decision-making regarding therapy; possible algorithm may include restricting the invasive procedure to patients with a high probability of no response or considering performing liver biopsy in non-responders after first course of treatment.
Pretreatment liver biopsy was recommended at the time the Canadian EAP was initiated.12-14 The current AASLD and Canadian guidelines continue to recommend liver biopsy prior to initiating treatment but both make the distinction that in genotype-2/3 treatment may be initiated without a pre-therapy liver biopsy.23,24
This current analysis substantiates the expert opinion that in genotype-2/3 patients, the pre-therapy liver biopsy does not make a difference in outcomes. A liver biopsy may be unnecessary in patients with genotypes 2/3 HCV infection, since more than 80% of them achieve SVR with the standard-of-care treatment.
In this analysis, genotype-1 patients achieved lower SVR rates with an absolute difference of 10.6% when pre-treatment liver biopsy was not performed. If we analyzed the number-needed-to-treat, there would be one additional subject achieving SVR for every 10 HCV patients starting therapy in the group with a pre-treatment liver biopsy. When we examined this more closely, with similar EVR and relapse rates, the difference in SVR rate was due to differences in EOTVR rate in genotype-1.
Of course there might have been some bias in the decision to biopsy or not biopsy prior to initiating treatment. Using the more rigorous multiple logistic regression analysis identified pre-treatment liver biopsy as independent association with SVR only in HCV-infected patients with genotype-1 but not with genotype-2/3.
We had hypothesized that if a treating physician suspected the patient was cirrhotic on the basis of clinical or radiological evidence, they may avoid a biopsy. However, in this analysis, surrogate markers of advanced liver disease or more resistance to treatment such as lower platelet counts, older age, higher BMI, and higher viral load were comparable between the groups that did or did not undergo liver biopsy before therapy. In fact, amongst those who underwent liver biopsy, 29.8% had advanced METAVIR fibrosis stages 3 or 4. This was no different from our own EAP phase-1 study, when liver biopsy was an inclusion criterion, where 34.2% had METAVIR fibrosis stages 3 or 4.21
This analysis excluded patients who had clear indications for advanced liver disease with portal hypertension (Child-Pugh class B or C, neutrophil count ≤ 1.5 x 109/L, and platelet count ≤ 90 x 109/L). It is likely that in those patients with stigmata of advanced liver disease or imaging compatible with cirrhosis or portal hypertension, treating physicians avoided pre-treatment liver biopsy. This would imply an over representation of cirrhotic patients in the group treated without a liver biopsy, and therefore, lower SVR that was seen in genotype-1 patients.
The interesting finding in the current analysis is that similar bias should have been expected to occur in the genotype-2/3 patients but yet similar SVR was achieved in the group undergoing pre-treatment liver biopsy vs. the group without pre-treatment liver biopsy.
Adherence is an important contributor to successful treatment of HCV. Both patient motivation and physician treatment experience may be important for a better adherence to combination treatment for HCV.25 Adherence of 85% or more to peginterferon and ribavirin treatment was associated with increased HCV suppression.26
In this analysis, close examination of adherence to treatment, revealed more dose adjustments in the group of patients who had cirrhosis on the pre-treatment liver biopsy but similar rates of dose discontinuation as the patients who had shown no cirrhosis on the liver biopsy.
Although data on patient’s motivation and treating physician’s experience were not collected, it is plausible that patients with pre-treatment liver biopsy, especially those with more advanced disease, may have had higher levels of commitment for completion of therapy; furthermore in those patients with known cirrhosis on the liver biopsy, treating physicians tried more dose adjustment rather than drug discontinuation to avoid failure and progression to end-stage liver disease.
Of course there is also the possibility that patients who did not undergo pre-treatment liver biopsy may have been less committed to starting therapy and to completion of longer course of therapy needed for genotype-1 patients but not impacting geno-type-2/3 patients significantly.
The finding that SVR rates were higher in genotype-1 patients treated after a biopsy does not resolve the ongoing debate about whether a biopsy is warranted for persons infected with HCV, genotype-1, whose response to such treatment approximates 50% among Caucasians and 30% among African Americans. A key question is whether a decision to treat with antiviral therapy is or is not altered by the information provided by histology.
In the past, advocates for routine biopsy prior to treatment argued that treatment should be provided to patients in whom the impact of treatment could be greatest, for example those with advanced fibrosis, rather than those patients who are less likely to benefit, such as those with minimal or no portal fibrosis. The main argument supporting this claim was the slowly progressive natural history of chronic hepatitis, with a median time from infection to cirrhosis of roughly 30 years.27
Today the rationale is to treat all patients to reduce the burden of liver disease. Often, physicians use the liver biopsy to support the decision to postpone therapy in a patient. With the newer HCV therapies in the pipeline, the requirement of liver biopsy prior to starting of treatment will have to be re-examined.
Opponents of routine liver biopsy question the view that liver-related deaths are the only serious consequences of HCV infection. Other benefits of viral elimination, such as a patient’s perceived lack of cleanliness, elimination of the risk of household and sexual transmission, and barriers to fertility, are usually ignored by academic committees, but are given a high priority by patients.28
Furthermore, treatment is less effective once fibrosis has progressed to a more severe stage,29 whilst even relatively mild disease can progress to a fibrotic event over years.30,31 Patients with mild hepatitis may not be at greatest risk for liver-related deaths, but might opt for treatment if available therapy offers a high probability of ridding them of their infection.
Since there has been significant advancements made in assessing liver fibrosis using elastography or serum fibrosis markers, either alone or in combination,32 some of these issues may be revisited with another cost-benefit analysis in genotype-1 treatment-naïve patients incorporating baseline information from a combination of non-invasive methods for analysis of liver histology vs. liver biopsy or considering liver biopsy only in non-responders to antiviral therapy
There are other reasons for considering liver biopsy in HCV patients such as identification of patients at high risk for development of hepatocellular carcinoma and large esophageal varices; these were not dealt with in this study though recent longitudinal data reveal that techniques such as transient elastography may be even more useful in identifying those with cirrhosis.33
ConclusionThe results of the present study show that genotype-1 patients who underwent liver biopsy had higher SVR rates than those who did not undergo biopsy. This might be related to better adherence over a longer duration to the combination therapy in more advanced liver disease.
This will require a controlled trial where genotype-1 patients would be randomized to antiviral therapy with or without pre-treatment staging of fibrosis (by liver biopsy or non-invasive markers).
In contrast liver biopsy had no impact on SVR rates in those infected with HCV genotype-2/3 challenging the assumed need for a biopsy in these individuals. These findings provide hard evidence for the recommendations published by recent consensus guidelines23,24,34-36 based only on expert-opinions.
AcknowledgementNadia Lesnikova and Jennifer Lee of Syreon Corporation, Vancouver, Canada assisted Dr. Balshaw with the initial data analyses. Mr. Chris Usaty, from Roche, Mississauga, Canada, was instrumental in coordinate all phases of development of this manuscript.
Disclosure StatementAs the guarantor of the article Dr. Peltekian accepts full responsibility for the conduct of analysis, had full access to the data and made the final decision to publish the article. The first draft of the Methods and Results and the tables were prepared by Blair J. Jarvis MSc under the direction of Dr. Pelte-kian using study reports generated by Syreon Corporation. Dr Peltekian prepared the first drafts of the Introduction, Discussion and the abstract. Mr. Jarvis did this work under contract to Roche Canada. Drs Peltekian, Bain, Lee, Sherman, Cooper, Yoshida, Marotta, Krajden, and Deschênes were involved in the planning and conduct of the study, collection and interpretation of data and revising the manuscript. Dr. Balshaw was involved in the design and conduct of the statistical analyses and drafting of the manuscript. All authors read and approved the final draft submitted to the journal. This study was funded by Roche, Mississauga, Canada.
Abbreviations- •
AASLD: American Association for the Study of Liver Disease.
- •
BMI: Body mass index.
- •
EAP: Expanded Access Program.
- •
EOTVR: End of therapy viral response.
- •
EVR: Early viral response.
- •
HCV: Hepatitis C virus.
- •
PCR: Polymerase chain reaction.
- •
RNA: Ribonucleic acid.
- •
SVR: Sustained viral response.
The Canadian Pegasys Study Group also includes: Frank Anderson MD, The Liver & Intestinal Research Centre, Vancouver, BC; Robert Bailey MD, Royal Alexandra Hospital, Edmonton, AB; Victor Feinman MD, Mount Sinai Hospital, Toronto ON; Susan Greenbloom MD, Toronto Digestive Disease Associates Inc., Toronto ON; Nir Hilzenrat MD, Montreal Jewish General Hospital, Montreal QC; Kelly Kaita MD, John Buhler Research Centre, Winnipeg MB; Linda Scully MD, The Ottawa Civic Hospital, Ottawa ON; Bernard Willems MD, CHUM Saint Luc Hospital, Montreal QC; Helga Witt-Sullivan MD, Hamilton Health Sciences Corp-General Site, Hamilton ON; Lawrence Worobetz MD, Royal University Hospital, Saskatoon, SK.